Session Information
Date: Saturday, November 6, 2021
Title: Epidemiology & Public Health Poster I: COVID-19 & Vaccination (0084–0117)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Little is known about the reactogenicity and related SARS-CoV-2 vaccine response in patients with chronic inflammatory disease (CID). While researchers have hypothesized increased symptomatology following vaccine would indicate a more robust vaccine response, this has not been demonstrated in the general population. Our objective was to characterize the adverse event (AE) profile in patients with CID receiving the SARS-CoV-2 vaccines and to better understand the relationship between reactogenicity and immunogenicity of the SARS-CoV-2 vaccines in patients with CID.
Methods: This study was part of a larger prospective study examining the immunogenicity and safety profile of the SARS-CoV-2 vaccines in patients with CID. Adults with CID and healthy controls eligible to receive the SARS-CoV-2 vaccine were enrolled. Subjects participated in 3 study visits (pre-vaccine, after dose 1, after dose 2) where blood and clinical data were collected. Assessment of AEs including local and systemic symptoms were solicited within 7 days of receiving each vaccine dose. Serum anti-SARS-CoV-2 spike (S) IgG+ antibody titerswere quantified to assess the magnitude of the humoral response following vaccination. Statistical analysis of solicited symptoms was performed utilizing two sample t-test and z-test. To study reactogenicity impact on vaccine antibody response, tobit regressions adjusting for patient status, age, gender, and vaccine type were utilized to account for left-censoring below the response detect limit.
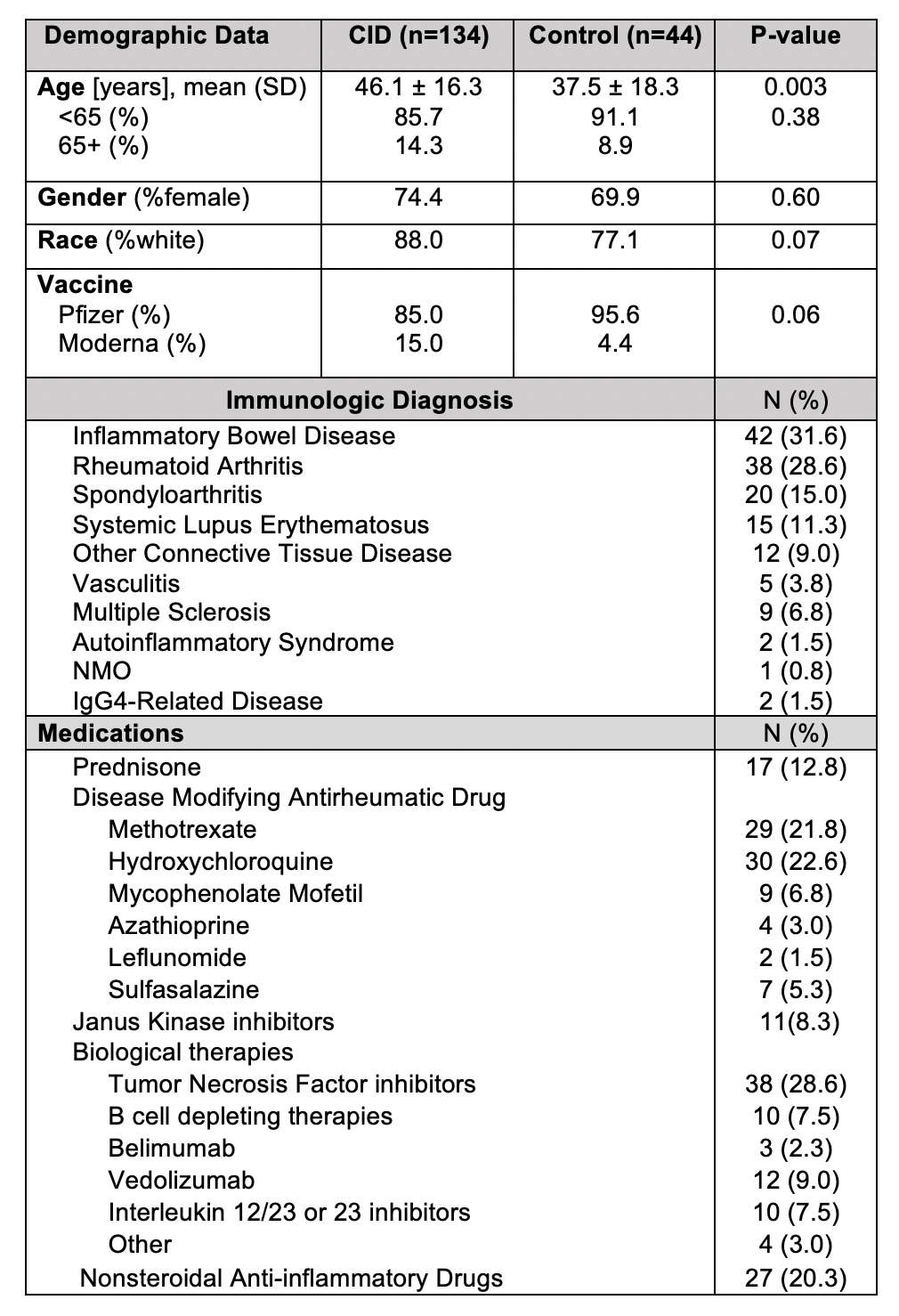
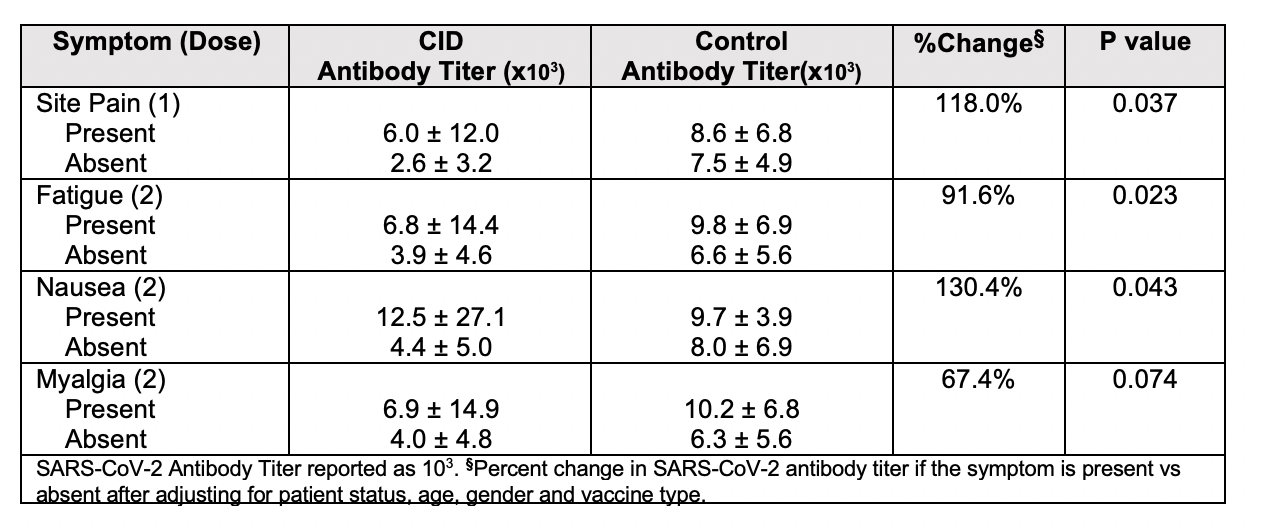
Results: 178 participants were included in this study including 134 patients with CID and 44 healthy controls. Demographic and clinical characteristics are shown in Table 1. Solicited symptoms among controls and CID patients are shown in Figure 1. CID patients experienced significantly more symptoms after the 1st dose of vaccine compared to controls (p=0.04), including more headache, myalgia and fatigue (p< 0.05). For immunogenicity, after adjustment for covariates, a higher number of reported symptoms after the second dose was associated with higher antibody titers (p=0.028). Each increase of one endorsed symptom was associated with 15.9% increase in antibody titer. Among all individual symptoms (Table 2), the most strongly associated at each dose included site pain after 1st dose (p=0.04) with 118% increase in antibody titer when present and fatigue after 2nd dose (p=0.02) with 91.6% change in antibody titer when present.
Conclusion: This study is one of the first to examine reactogenicity of the novel SARS-CoV-2 vaccines among patients with CID and the relationship between reactogenicity and vaccine response. We demonstrated that patients with CID have a distinct reactogenicity profile from their healthy control counterparts with CID patients having more numerous symptoms following 1st dose of vaccine. Furthermore, we demonstrated an association between reactogenicity and immunogenicity in CID patients. This finding, thus far not seen in the general population, may speak to the more variable immunogenicity in patients with CID and could be an important indicator of vaccine response to the novel SARS-CoV-2 vaccines.
To cite this abstract in AMA style:
Yang M, E. Taylor K, Paez D, Carividi A, Demissie E, Pawar N, El-Qunni, A, McMorrow L, Schriefer R, Huang K, Kinnett B, Kim W, Ellebedy A, Ciorba M, Paley M, Deepak P, Kim A, Katz P, Matloubian M, Nakamura M, Gensler L. Reactogenicity of the SARS-CoV-2 Vaccines Associates with Immunogenicity in Patients with Autoimmune and Inflammatory Disease [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/reactogenicity-of-the-sars-cov-2-vaccines-associates-with-immunogenicity-in-patients-with-autoimmune-and-inflammatory-disease/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reactogenicity-of-the-sars-cov-2-vaccines-associates-with-immunogenicity-in-patients-with-autoimmune-and-inflammatory-disease/