Session Information
Date: Sunday, November 17, 2024
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Unlike in many other disease states, there is no accepted definition for clinical response in lupus nephritis (LN). The Phase 3 AURORA 1 study of voclosporin (VCS) used complete renal response at 52 weeks (CRR; UPCR ≤0.5 g/g, stable eGFR, low-dose steroids, and no rescue medications) as the primary efficacy endpoint.1 Significantly more patients treated with VCS in combination with MMF and low-dose glucocorticoids (GCs) achieved CRR compared to patients in the control arm treated with MMF and low-dose GCs (41% vs 23%, odds ratio [OR] 2.65, 95% confidence interval [CI] 1.64-4.27, p< 0.0001). Patients who completed AURORA 1 and elected to continue treatment in the AURORA 2 continuation study demonstrated maintained efficacy and stable renal function up to 36 months.2
In 2024, following the completion of AURORA 2, the US FDA compared the efficacy benefit of VCS to control using a new longitudinal endpoint, sustained complete renal response (SCRR), defined as achieving CRR at Month 12 of AURORA 1 and maintaining the response at each subsequent study visit through the end of AURORA 2 (Month 36). This new endpoint was incorporated into the recently updated labeling for VCS, and results of the analysis are presented here.3
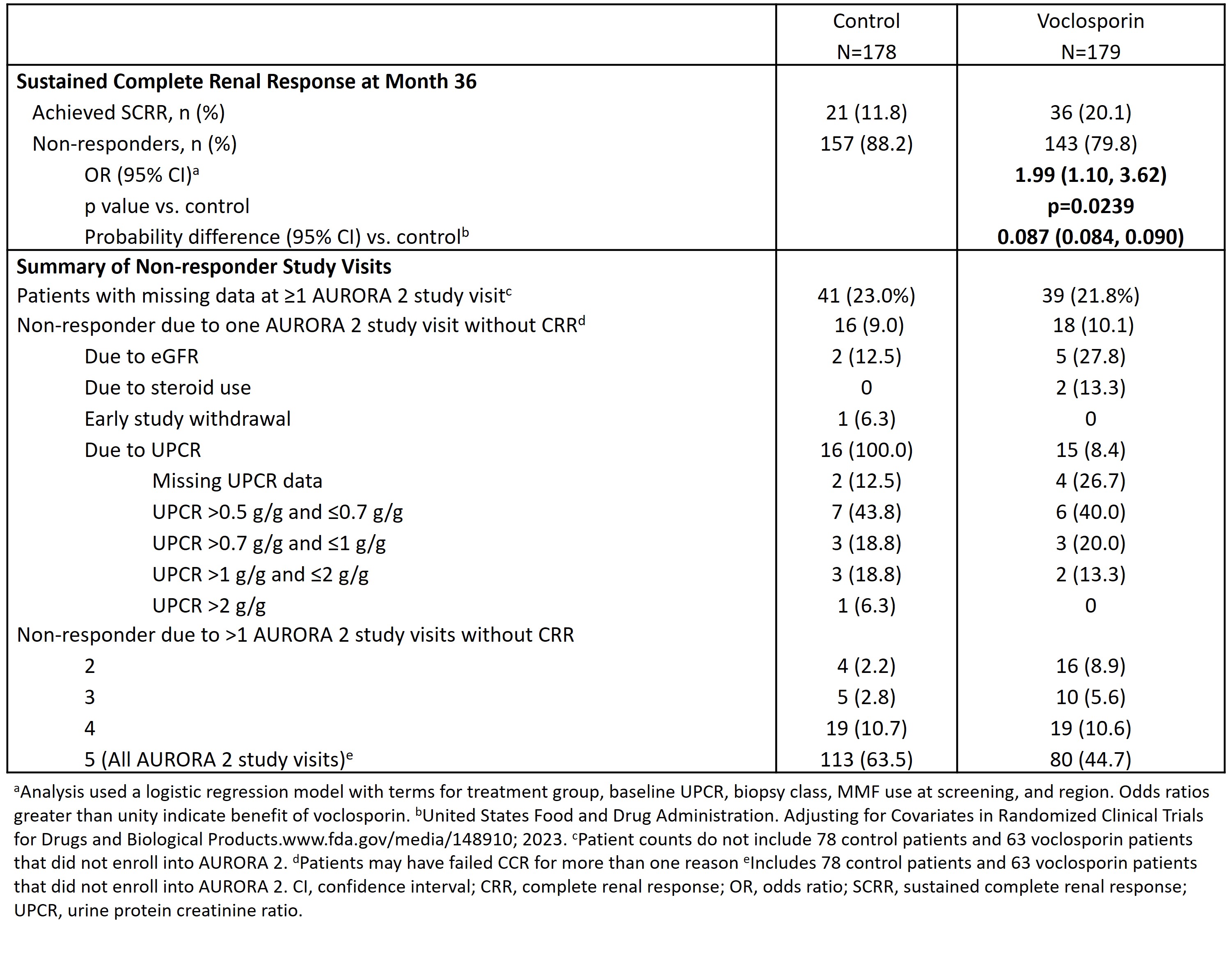
Methods: Patients completing AURORA 1 were eligible to enter AURORA 2 on the same blinded therapy (VCS or placebo) combined with MMF (target of 2 g/d) and GCs (20-25 mg/day tapered to 2.5 mg/d by Week 16) for an additional two years. Only patients who continued in AURORA 2 (N=216) were analyzed for SCRR, although all patients who initiated treatment in AURORA 1 (N=357) were included in the denominator of the efficacy analysis. Patients with missing data at AURORA 2 study visits were considered non-responders.
Results: Of the 179 VCS- and 178 control-treated patients enrolled in AURORA 1, 116 (64.8%) and 100 (56.2%) patients, respectively, continued treatment in AURORA 2. Of these patients, 39 (21.8%) and 41 (23.0%), respectively, had missing data and were considered non-responders. At Month 36, 36 (20.1%) of 179 VCS-treated patients and 21 (11.8%) of 178 control-treated patients achieved SCRR (OR 1.99, 95% CI 1.10, 3.62; p=0.0239), with a probability difference between the two groups of 0.087 (95% CI 0.084, 0.09). Additionally, 10.1% of VCS and 9.0% of control-treated patients achieved CRR at all but one study visit (Table 1).
Conclusion: SCRR is a novel endpoint that can be leveraged to illustrate disease trajectory of LN and treatment efficacy. Consistent with using CRR as an efficacy endpoint in AURORA 1, more VCS- than control-treated patients achieved SCRR, despite nearly 40% of the analyzed study population being ineligible due to the voluntary nature of AURORA 2 participation. The stringency and potential utility of this new endpoint require further exploration.
This work was funded by Aurinia Pharmaceuticals Inc.
1. Rovin BH, et al. The Lancet. 2021; May 29;397(10289):2070-2080. DOI: 10.1016/S0140-6736(21)00578-X.
2. Saxena A, et al. Arthritis Rheumatol. 2024; Jan;76(1):59-67. doi: 10.1002/art.42657. Epub 2023 Sep 15.
3. LUPKYNIS [package insert]. Rockville, MD: Aurinia Pharma US, Inc.; 2021. Revised: 4/2024.
To cite this abstract in AMA style:
Yap E, Truman M, Auguste C, Birardi V, Keenan G. Rates of Sustained Complete Renal Response with Long-term Use of Voclosporin in AURORA 2 [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/rates-of-sustained-complete-renal-response-with-long-term-use-of-voclosporin-in-aurora-2/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rates-of-sustained-complete-renal-response-with-long-term-use-of-voclosporin-in-aurora-2/