Background/Purpose: Over the past 40 years failures to achieve remission in a proportion of patients and the potential development of significant adverse effects have been major drawbacks of cyclophosphamide (CYC)-based regimens. Rituximab, an anti-CD20 monoclonal antibody, has compared favorably to CYC-based regimens for remission-induction of ANCA-associated vasulitis (AAV) in controlled clinical trials. It has also been able to maintain clinical remission in some patients for several months of follow up without the need for medication-based maintenance regimens. We intend to describe the rates of major adverse events (malignancy, infections) with rituximab and control interventions in existing randomized controlled trials for the treatment of AAV.
Methods: All randomized controlled trials of rituximab in patients with AAV were sought in PubMed, EMBASE, and Cochrane databases during December 2012. Pooled treatment effects were estimated using risk ratio (RR) with the Mantel–Haenszel risk ratio in a random-effects model. Heterogeneity was assessed using chi-square tests and I2 statistic; we defined I2 < 25% as low heterogeneity according to the Cochrane Handbook of Systematic Reviews. For statistical analysis, we used Review Manager 5.1
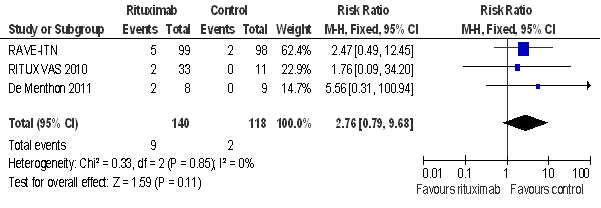
Results: Upon analysis of the pooled data of included trials [1, 2, 3], rituximab was shown to be more efficacious in achieving remission than the control interventions. Even though no statistically significant difference was found in the rates of adverse events, the rate of malignancy tended to be more prevalent in the rituximab group when compared to the control group (P= 0.85, figure 1). In contrast, infection rates were more prevalent in the control group when compared to rituximab (P= 0.84, figure 2). Patient exposure to several immunosuppressive drugs in these trials, makes appraising the direct responsibility of one agent, rituximab or CYC, in the occurrence of the adverse events reported by investigators a significant challenge. In addition, AAV itself may predispose patients to malignancy or infection. Long-term follow up of the patients from these trials may provide further information about the safety profile of rituximab.
Conclusion: Rituximab has been shown an effective alternative for achieving remission in AAV when compared to the control interventions in this meta-analysis. This therapy may allow long term disease control with fewer side effects in patients with AAV but more randomized control trials are needed.
Figure 1: Malignancy rates
Figure 2: Infection rates
Disclosure:
C. Mejia-Otero,
None;
C. J. Lozada,
None;
L. Arias-Urdaneta,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rate-of-infection-and-development-of-malignancy-in-patients-with-anca-associated-vasculitis-treated-with-rituximab-a-meta-analysis-from-randomized-trials/