Session Information
Date: Monday, October 27, 2025
Title: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Belimumab (BEL), a B-cell modulator mAb that selectively inhibits soluble B-lymphocyte stimulator and reduces autoreactive B cells that drive lupus disease activity, is approved for SLE and LN in adults and children.1 Prolonged oral glucocorticoid (OGC) use can lead to organ damage, and OGC reduction is an important treatment goal in SLE. BEL has been shown to reduce OGC use in the real world; however, limited evidence exists regarding the characteristics of patients who are able to reduce OGC use with BEL. We studied OGC reduction or discontinuation within 6 months of BEL initiation in patients with SLE in US clinical practice and aimed to identify patient characteristics that may support prediction of early OGC dose reduction or discontinuation.
Methods: This retrospective, longitudinal cohort study (GSK Study 222157) used health record data from the Specialty Networks rheumatology practice database. Eligible patients were aged ≥18 years, diagnosed with SLE, initiated either subcutaneous (SC) or intravenous (IV) BEL (9/1/2011–4/25/2024; defined index), had ≥12 months of pre-index (defined as baseline period) and ≥10 months of post-index clinical activity, ≥1 OGC prescription with dose information (in prednisone-equivalent units), and no OGC discontinuation during baseline. To ensure sufficient exposure, within 3 months of index prescription, SC BEL initiators were required to have ≥1 SC or ≥1 IV BEL record while IV BEL initiators were required to have ≥1 SC or ≥2 IV BEL records. OGC dose reduction by ≥50% or discontinuation (≥120 consecutive days without OGC supply) within 6 months was defined as “early” if it occurred within the first quartile of the time from index to either event calculated across all patients, using a data-driven approach. Predictors of early OGC dose reduction or discontinuation were identified using a least absolute shrinkage and selection operator (LASSO) logistic regression model. Candidate predictors included age, sex, race, year of index, SLE disease severity, organ damage, Quan-Charlson Comorbidity Index, and comorbidities.
Results: Among 541 patients included in the study, approximately half (50.8%, n=275) achieved ≥50% OGC dose reduction or discontinuation within 6 months of initiating BEL. Median time to ≥50% dose reduction or discontinuation was 177 days and 25% (n=136) of patients achieved this outcome in < 2 months (57 days). Patients with early (i.e. within 57 days) OGC reduction or discontinuation were slightly older than those without (50.3 vs 48.3 years), a greater proportion had moderate (74.3% vs 66.9%) or severe (3.7% vs 2.2%) SLE, but the same proportion (9.6%) had evidence of baseline organ damage (Table 1). LASSO regression identified moderate/severe SLE at baseline (vs mild) (odds ratio [95% confidence interval]: 1.64 [1.04, 2.65]) as a predictor of early OGC dose reduction or discontinuation (Table 2).
Conclusion: Among patients initiating BEL treatment for SLE, half achieved OGC discontinuation or dose reduction by ≥50% within 6 months. This outcome was achieved by 25% of patients within 2 months of BEL initiation, with this earlier benefit more likely in patients who had moderate/severe SLE. Funding: GSK Reference 1GSK. Benlysta US prescribing information. 2024
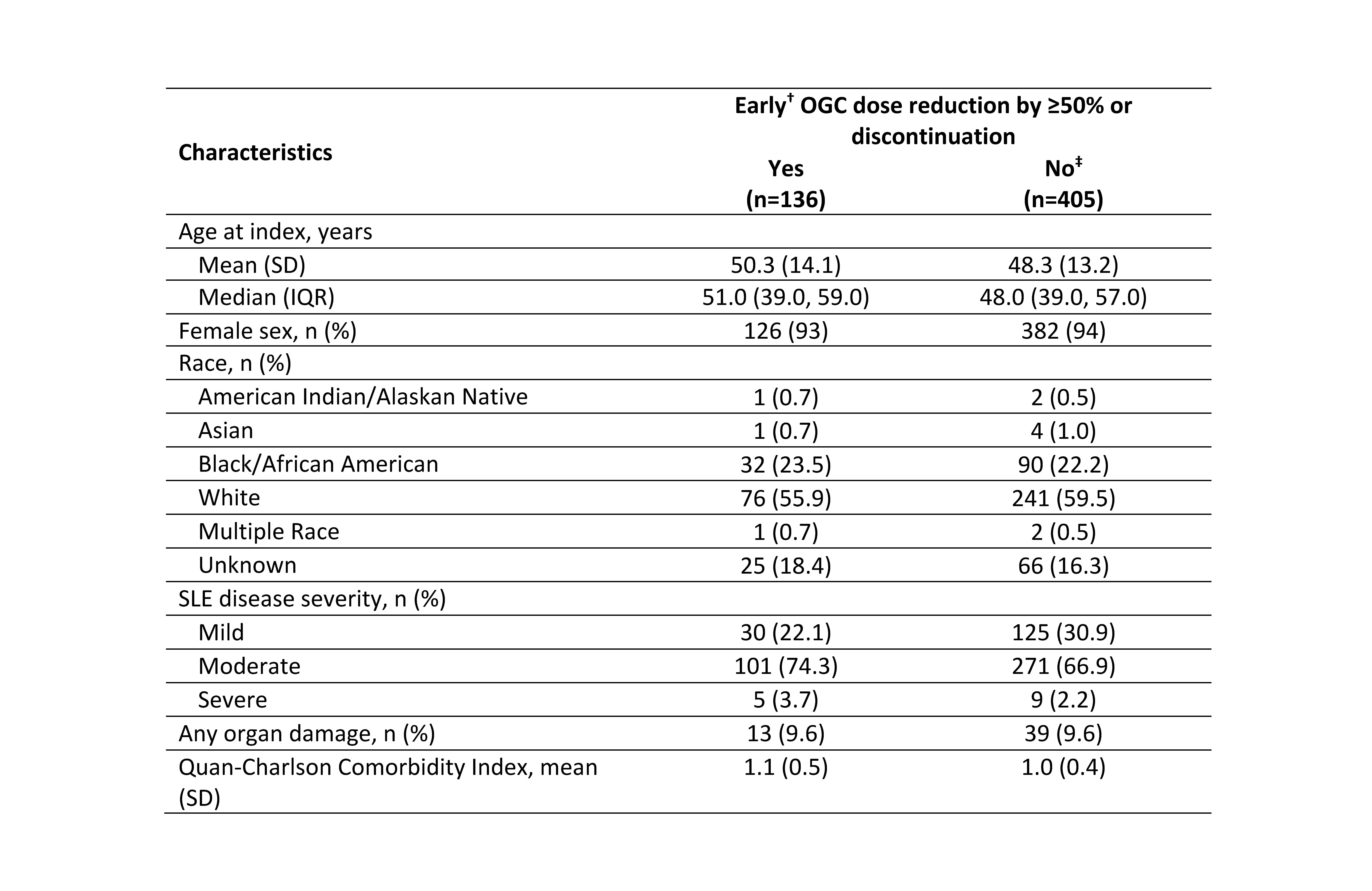 Table 1. Patient baseline demographics and clinical characteristics by early OGC dose reduction by ≥50% or discontinuation*.
Table 1. Patient baseline demographics and clinical characteristics by early OGC dose reduction by ≥50% or discontinuation*.
*≥50% OGC dose reduction was defined as ≥50% decrease in the average daily dose of OGC from baseline, and discontinuation of OGC was defined for a patient who was expected to be using an OGC as of the index date and did not have another OGC prescription for >120 days after the end of the days’ supply of the previous OGC prescription; †within 57 days post-index; ‡this group includes patients who reached OGC dose reduction by ≥50% or discontinuation more than 57 days post-index and patients who did not reach this outcome.
IQR, interquartile range; SD, standard deviation.
.jpg) Table 2. LASSO-selected predictors of early OGC dose reduction or discontinuation*.
Table 2. LASSO-selected predictors of early OGC dose reduction or discontinuation*.
*ORs and 95% CIs were estimated from a logistic regression model using only covariates selected by the LASSO procedure. The tuning parameter that results in the lowest deviance was chosen for the LASSO procedure; †within 57 days post-index.
CI, confidence interval; OR, odds ratio.
To cite this abstract in AMA style:
Chen Y, DerSarkissian M, Rui S, Clark J, Moldaver D, Ellis J, Worley K, Patel A. Rapid Oral Glucocorticoid Discontinuation or Dose Reduction Among US Patients with SLE Receiving Belimumab in a Real-World Setting [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/rapid-oral-glucocorticoid-discontinuation-or-dose-reduction-among-us-patients-with-sle-receiving-belimumab-in-a-real-world-setting/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rapid-oral-glucocorticoid-discontinuation-or-dose-reduction-among-us-patients-with-sle-receiving-belimumab-in-a-real-world-setting/
