Session Information
Date: Wednesday, November 15, 2023
Title: Abstracts: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science II
Session Type: Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Interferon (IFN) dysregulation is a key feature in the pathogenesis of Dermatomyositis (DM). PF-06823859 is a potent, selective, humanized IgG1 neutralizing antibody directed against IFNb. We examined the onset of efficacy of PF-06823859 in patients (pts) with moderate-to-severe refractory DM.
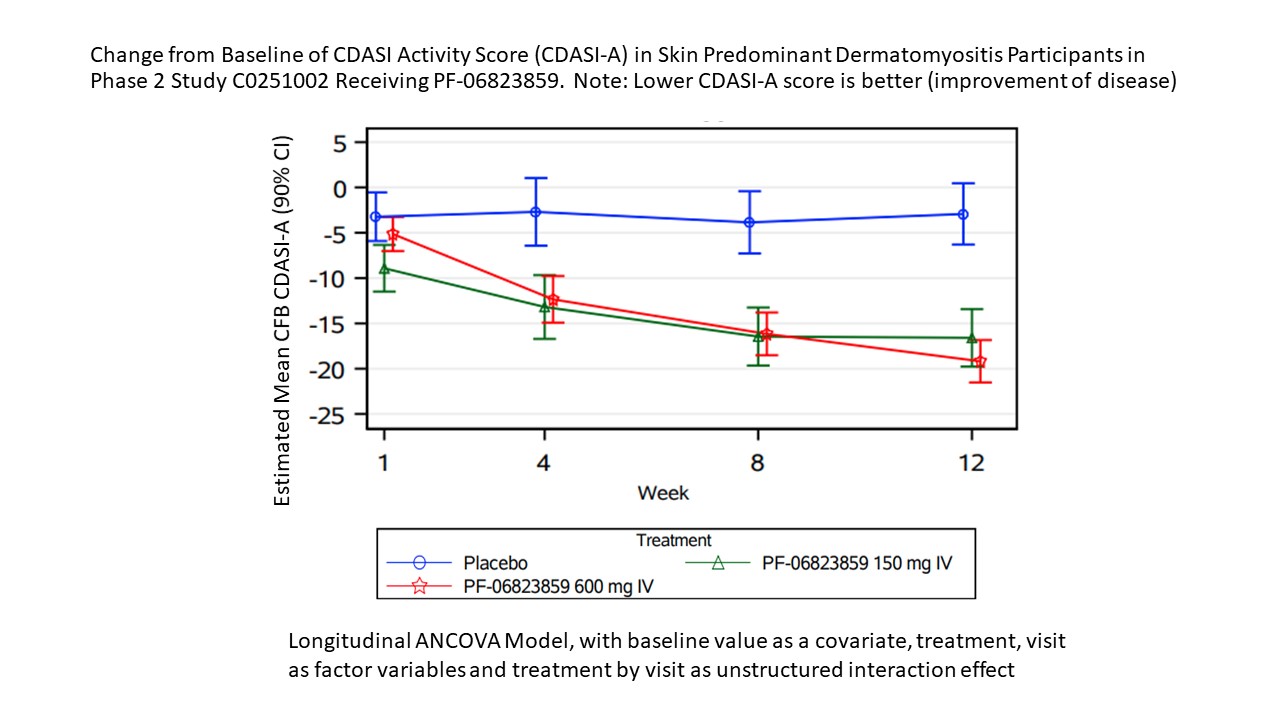
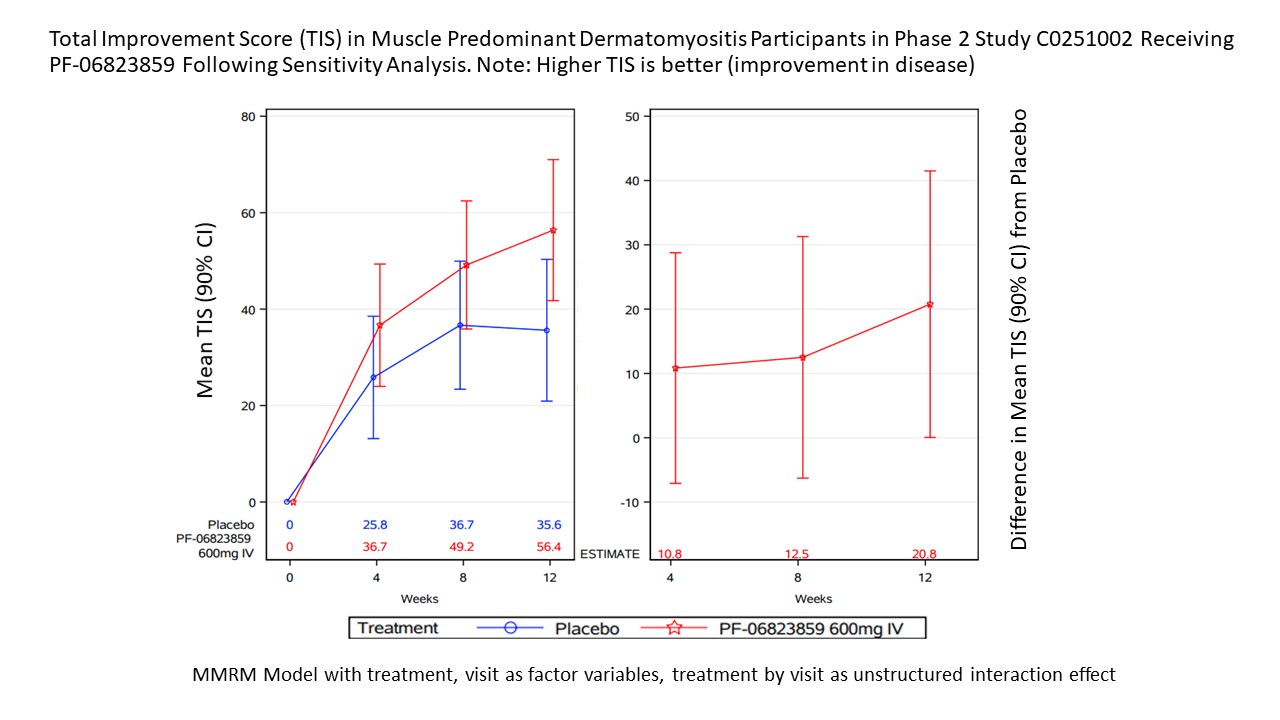
Methods: This double-blind, placebo (PBO)-controlled, Phase 2 study (NCT03181893) enrolled adult pts (18–75 years) with moderate-to-severe refractory DM.Pts with skin disease predominant (SP) DM and Cutaneous Dermatomyositis Disease Area and Severity Index activity [CDASI-A] score ≥14 who had failed ≥1 standard of care systemic treatment were randomized to PBO, PF-06823859 150 mg or 600 mg (intravenous administration on Day 0, wks 4 and 8). Pts with muscle disease predominant (MP) DM who met 1 of the following: (1) Manual Muscle Testing (MMT-8) ≤136/150 and Physician Global Assessment (PhGA) ≥3 cm on a 0–10 cm visual analogue scale (VAS) or (2) sum of PhGA, Patient Global Assessment (PtGA), Extra-muscular Global Assessment ≥10 cm (0–10 cm VA for each) and refractory disease, were randomized to PBO or IV PF-06823859 600 mg(Day 0, wks 4 and 8). Onset of efficacy was evaluated in the SP cohort using the CDASI-A, 5D-itch, Dermatology Quality of Life Index (DLQI), and SF-36 change from baseline to Wk 12. In the MP cohort, onset of efficacy was evaluated using the Total Improvement Score (TIS), Manual Muscle Testing (MMT-8), creatine kinase (CK) levels, PtGA, Health Assessment Questionnaire Disability Index (HAQ-DI) and FACIT-Fatigue change from baseline to Wk 12.
Results: SP and MP cohorts had 57 and 18 pts respectively. In the SP cohort, differentiation from PBO in CDASI-A was observed at Wk 4 for both 150 mg (PBO-adjusted CFB -13.19 [p=0.0006]) and 600 mg (PBO-adjusted CFB -12.35 [p=0.0004]) doses. A reduction in CFB 5D-itch was observed for the 600 mg dose at Wk 1 (PBO-adjusted CFB 5D-itch 600 mg [p=0.0359]). PBO-adjusted CFB in DLQI and SF-36 mental component were observed for 150 and 600 mg (p=0.024, p=0.016 and p=0.009, p=0.0398, respectively) at Wk 4. In the MP cohort, a numerical advantage was observed in TIS scores at 600 mg compared to PBO with increasing trends from Wks 4-12 (PBO-adjusted CFB [p=0.0497] at Wk 12) following a sensitivity analysis that removed affected data following use of a prohibited medication by one pt. Significant reduction in CK and HAQ-DI was observed at Wk 4 and 8, respectively (PBO-adjusted CFB for 600 mg CK [p=0.044] and HAQ-DI [p=0.035]). A numerical differentiation in PtGA started at Wk 1 and reached significance at Wk 8 (PBO-adjusted CFB 600 mg [p=0.0470]). A numerical advantage in MMT-8 and FACIT-F were observed starting at Wk 4, but did not reach statistical significance.
Conclusion: PF-06823859 induced rapid improvement in 5D-itch (Wk 1), followed by CDASI-A, DLQI, and SF-36 (mental component) at Wk 4 in DM pts with SP disease. Rapid improvement in MP DM was observed at Wk 1 for PtGA, and at Wk 4 for MMT-8 and CK. The inhibition of IFNβ activity by PF-06823859 is a promising treatment in DM, with a more rapid onset of efficacy potentially allowing a faster taper of steroids than current DM therapies.
To cite this abstract in AMA style:
Aggarwal R, Peeva E, Mangold A, Sloan A, Chu M. Rapid Onset of Response in Adult Dermatomyositis Patients Receiving Anti-interferon β (PF-06823859): Results of a Phase 2, Double-blind, Randomized, Placebo-Controlled Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/rapid-onset-of-response-in-adult-dermatomyositis-patients-receiving-anti-interferon-%ce%b2-pf-06823859-results-of-a-phase-2-double-blind-randomized-placebo-controlled-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rapid-onset-of-response-in-adult-dermatomyositis-patients-receiving-anti-interferon-%ce%b2-pf-06823859-results-of-a-phase-2-double-blind-randomized-placebo-controlled-study/