Session Information
Date: Sunday, November 7, 2021
Title: Systemic Sclerosis & Related Disorders – Basic Science Poster (0541–0559)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Systemic sclerosis (SSc) is an autoimmune fibrotic disease with unclear pathogenesis and no effective therapies. Increased proportion of CD163-positive, profibrotic macrophages has been described in SSc, but its contribution to SSc pathogenesis is unclear. Activation of the mammalian target of Rapamycin (mTOR) pathway has been linked to SSc fibrosis, and was also previously shown to induce a profibrotic phenotype in macrophages. We have recently demonstrated that Fli1, a member of the Ets family of transcription factors, is underexpressed in SSc myeloid cells. Co-culture of human dermal fibroblasts and Fli1 depleted myeloid cells resulted in potent induction of CD163 and other profibrotic genes in myeloid cells, and abundant collagen deposition by fibroblasts, which was independent of TGFβ pathway activation.1 The aim of our study was to evaluate the mechanism by which deletion of Fli1 in myeloid cells exerts its profibrotic effects.
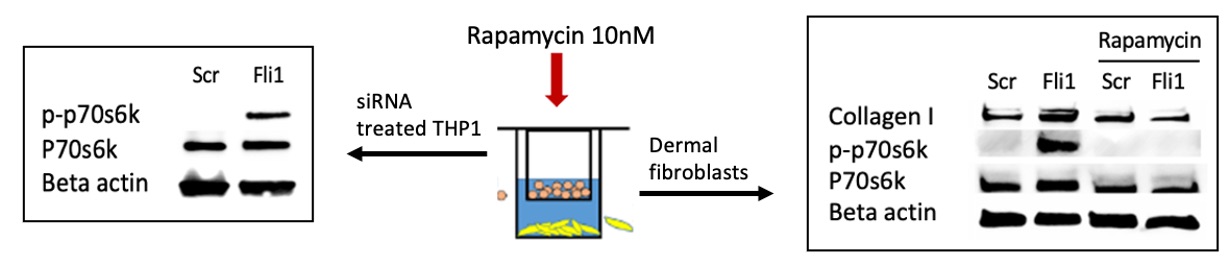
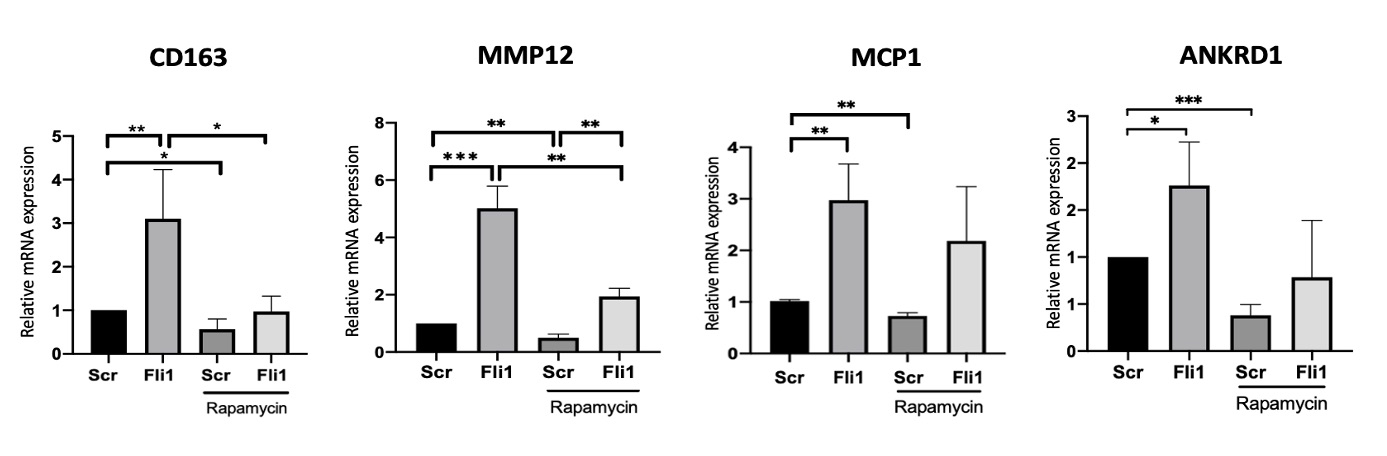
Methods: Primary human dermal fibroblasts were isolated from skin biopsies and were co-cultured with THP1 cells using inserts. Fli1 was depleted via siRNA in THP1 cells using Lipofectamine, and Rapamycin (10nM) was used to treat the co-cultures. Western blot was used to assess the protein levels of collagen and P-p70S6K (Thr389), and qRT-PCR was used to measure mRNA expression of CD163, MCP1, MMP12 and ANKRD1.
Results: Co-culture of human dermal fibroblasts and Fli1 depleted macrophages resulted in activation of the mTOR pathway, with increased phosphorylation of p70S6K (Thr389) in THP1 cells and dermal fibroblasts. Rapamycin treatment blocked the increase in collagen deposition by fibroblasts cocultured with Fli1 depleted myeloid cells (Fig.1). Furthermore, blockade of the mTOR pathway using Rapamycin in THP1 cells alone, in the presence or absence of siRNA against Fli1, reversed the effects of Fli1 inhibition on the mRNA levels of CD163, MCP1, MMP12 and ANKRD1 in these cells (Fig.2).
Conclusion: Fli1 deletion in myeloid cells triggers activation of a profibrotic phenotype, with increased fibroblast collagen deposition, via activation of the mTOR signaling pathway. Myeloid Fli1 deficiency may contribute to SSc pathogenesis, and Rapamycin may be a potential therapeutic option for SSc fibrosis.
To cite this abstract in AMA style:
El-adili F, Marden G, Trojanowska M, Bujor A. Rapamycin Blocks the Profibrotic Effects of Fli1 Downregulation in Scleroderma Myeloid Cells [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/rapamycin-blocks-the-profibrotic-effects-of-fli1-downregulation-in-scleroderma-myeloid-cells/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rapamycin-blocks-the-profibrotic-effects-of-fli1-downregulation-in-scleroderma-myeloid-cells/