Session Information
Date: Sunday, October 21, 2018
Title: Rheumatoid Arthritis – Treatments Poster I: Strategy and Epidemiology
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: SB4, SB2, and SB5 are biosimilars of reference etanercept, infliximab, and adalimumab, respectively. Radiographic data were assessed using the modified Total Sharp Score (mTSS) at week 0 and final week (week 52 for etanercept and adalimumab and week 54 for infliximab) in phase III biosimilar studies.1-3 The purpose of this analysis is to identify baseline demographics and disease characteristics that are associated with radiographic progression (RP) and build a matrix model to show the proportion of patients with RP by the identified baseline factors.
Methods: Patients who had radiographic data were pooled. RP was defined as a change in mTSS >0 from week 0 to final week. The three baseline factors that had the strongest Pearson¡¯s correlation coefficient with the change in mTSS were selected, and univariate logistic regression analysis was performed to assess the association between each baseline factor and predicted probability of patients with RP. Then, multivariate logistic model was fitted to develop a matrix that shows the proportion of patients with RP in each tertile of baseline factors.
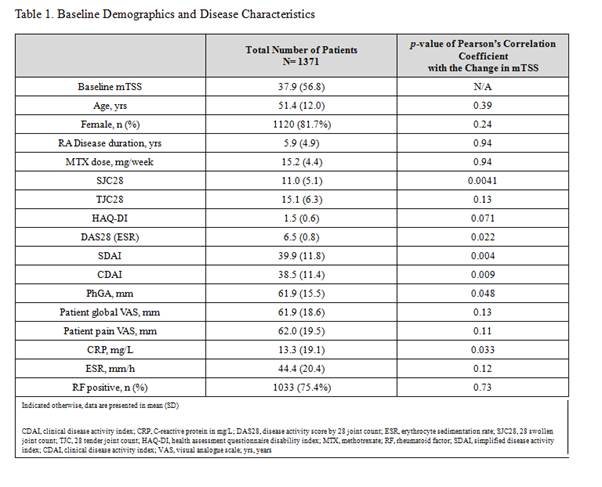
Results: A total of 1371 patients had radiographic assessments. The mean change in mTSS was 0.41 and the number of patients who had RP was 376 patients (27.4%). The swollen joint count 28 ([SJC28], p-value = 0.0041), C-reactive protein ([CRP], p-value = 0.033), and Physician Global Assessment ([PhGA], p-value = 0.048) had the strongest correlation with RP (Table 1). Predicted probability of patients with RP as a function of SJC28, CRP, and PhGA is displayed in Figure 1. The proportion of patients with RP increased as baseline levels of each factor worsened (Figure 2). The proportion of patients with RP was greatest (37%, 95% CI: 32, 43) in the highest tertile of each category (SJC28 >17, CRP >30, and PhGA >80) and least (12%, 95% CI: 8, 20) in the lowest tertile of each category (SJC28< 10, CRP <5, and PhGA <50).
Conclusion: A pooled radiographic assessment data showed that baseline SJC28, CRP, and PhGA are associated with RP, and the proportion of patients with RP increases as each baseline factor worsened.
References
- Smolen JS et al. Rheumatology. 2017 Oct 1;56(10):1771-1779
- Weinblatt ME et al. Arthritis Rheumatol. 2018; Jan;70(1):40-48
- Weinblatt ME et al. Arthritis Rheumatol. doi: 10.1002/art.40444
To cite this abstract in AMA style:
Smolen JS, Kang YM, Yoo WH, Emery P, Weinblatt ME, Keystone EC, Genovese MC, Myung G, Hong E, Baek I, Ghil J. Radiographic Progression Based on Baseline Demographics and Disease Characteristics from Three TNF-Alpha Inhibitor Biosimilar Studies in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/radiographic-progression-based-on-baseline-demographics-and-disease-characteristics-from-three-tnf-alpha-inhibitor-biosimilar-studies-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/radiographic-progression-based-on-baseline-demographics-and-disease-characteristics-from-three-tnf-alpha-inhibitor-biosimilar-studies-in-patients-with-rheumatoid-arthritis/