Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Healthcare disparities exist in patients living with rheumatologic diseases. Factors contributing to disparities include age, sex, race, or sociodemographic variables, each playing a crucial role in determining equitable access. Race is being recognized as a core factor in healthcare inequity. To this end, the path to healthcare equity would start with adequate racial representation in the clinical trials. This study was undertaken to analyze the racial representation in rheumatology clinical trial study population over a period of 20 years.
Methods: We utilized ClinicalTrials.gov, a US-based registry of clinical trials, run by the United States National Library of Medicine at the National Institute of Health (NIH). Clinical trials in Rheumatology were filtered using the following criteria in advanced search: Disease, Study type: Interventional (Clinical trial), Study results: Studies with results, Status: Completed, Location: United States, Phase: 3 or 4, First Posted: 01/01/2002, Last update posted: 01/01/2022. Five common rheumatologic diseases were analyzed, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic sclerosis (SSc) spondyloarthritis (SpA), and gout. Data were collected in an Excel spreadsheet and descriptive statistics were used for analysis. A comparison of racial representation was made with the US census 2020 data.
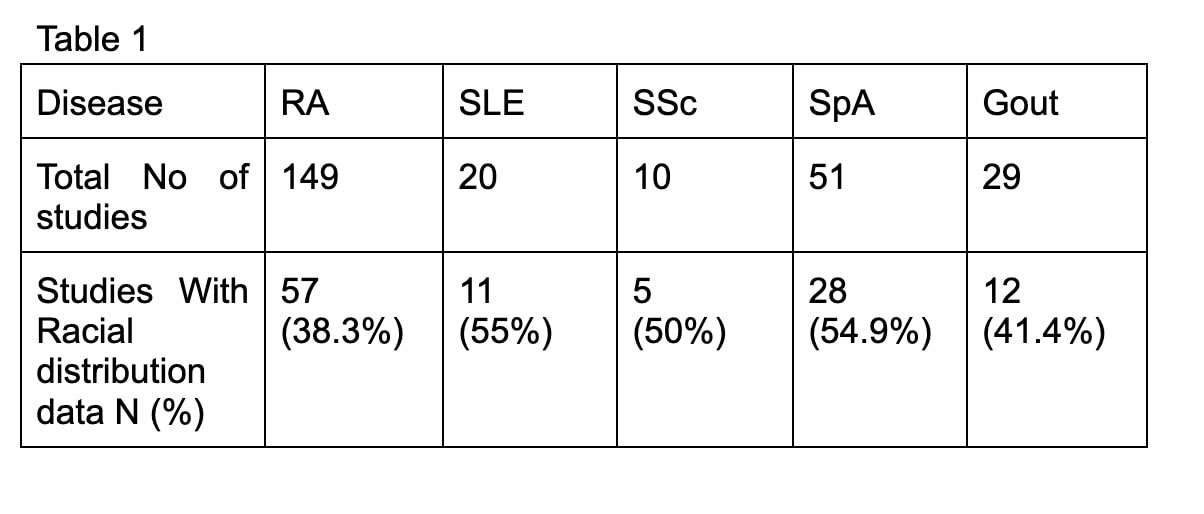
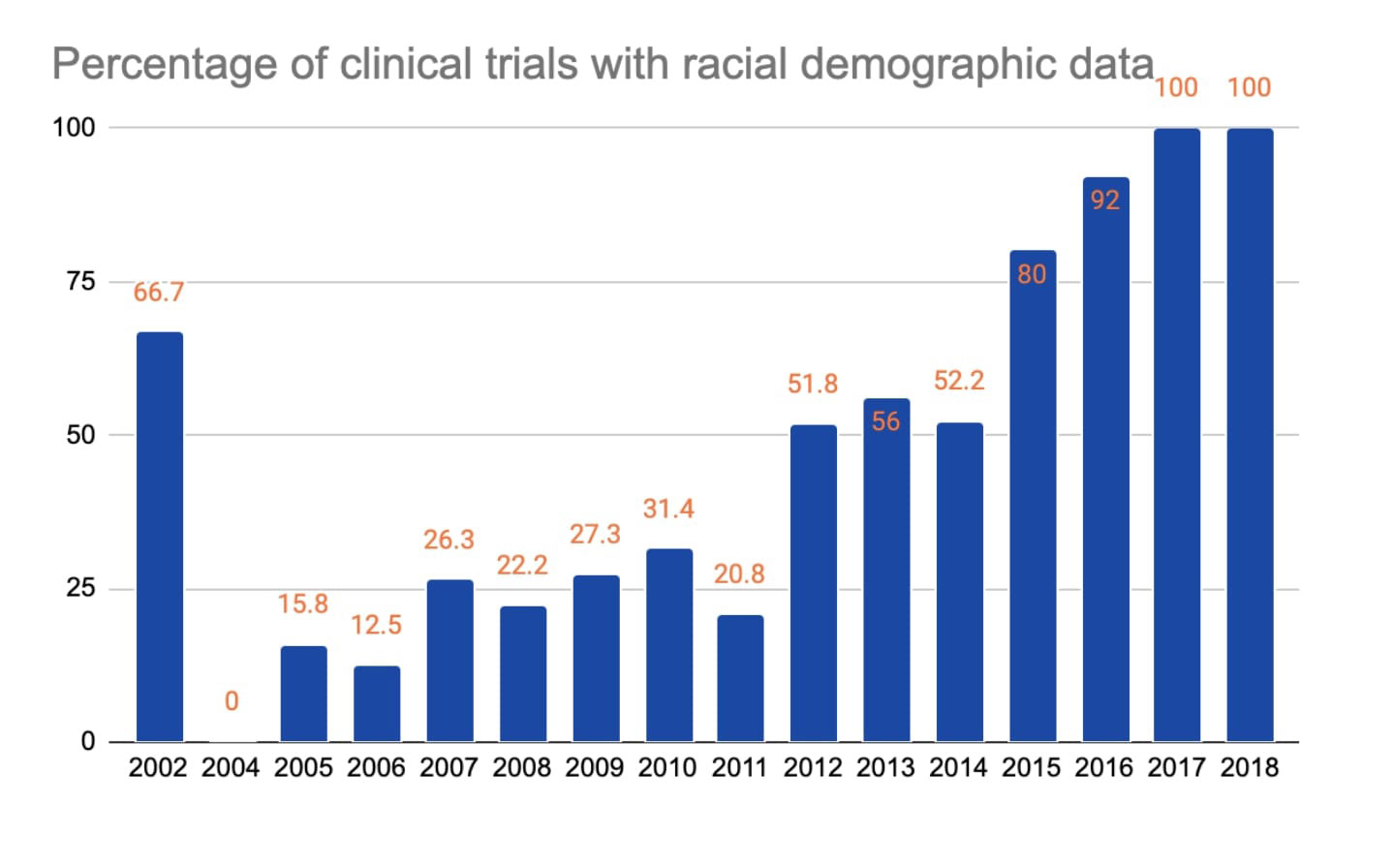
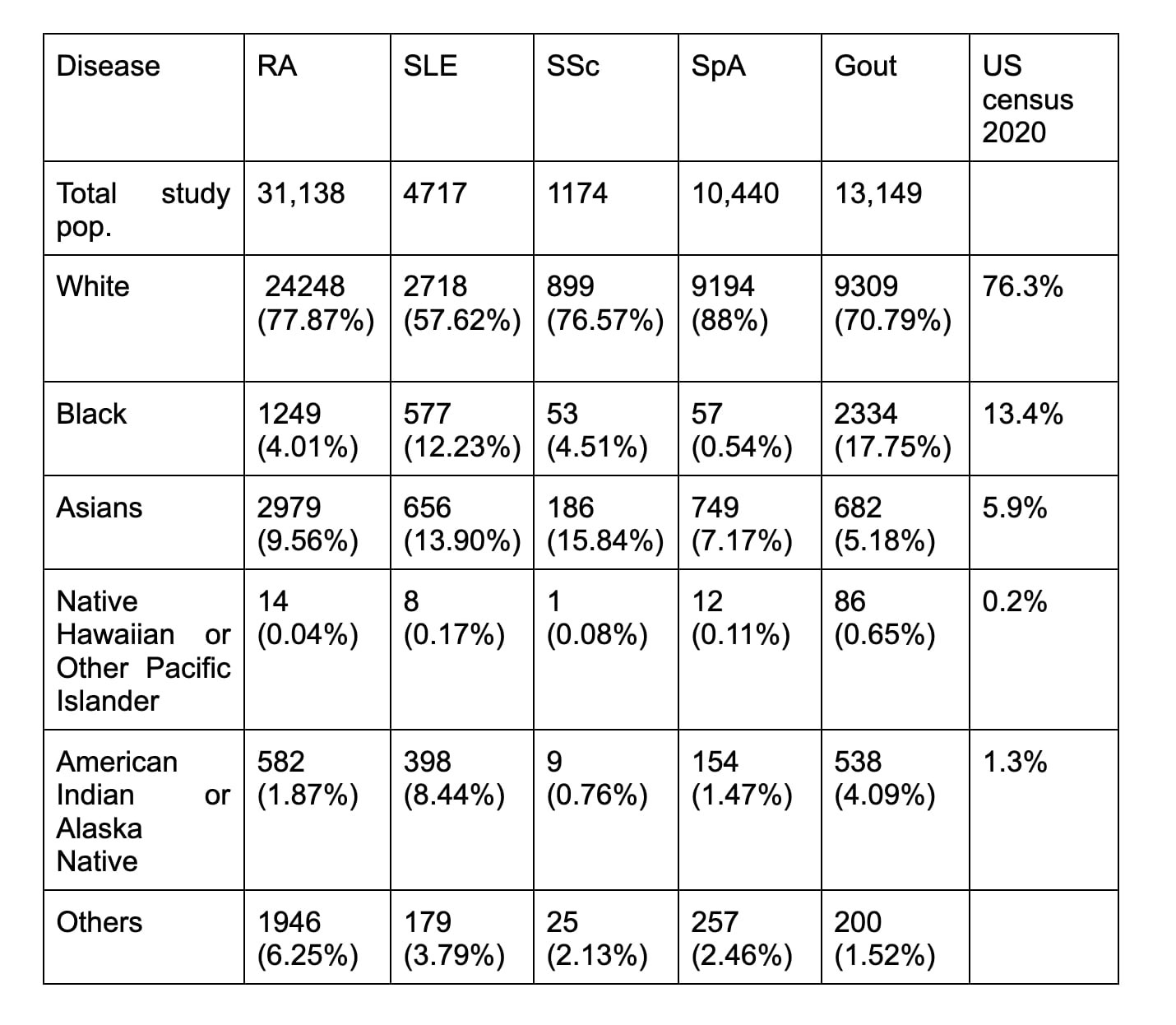
Results: 259 studies met inclusion criteria out of which 113 studies (43.6%) of rheumatologic clinical trials were disclosing racial demographic data. Table 1 provides percentage of clinical trials with racial demographic information by disease: RA 57/149 (38.3%), SLE 11/20 (55%), SSc 5/10 (50%), SpA 28/51 (54.9%), and gout 12/29 (41.4%). Graph 1 shows the growing percentage of clinical trials reporting racial demographic data by the year reaching 100% in 2017 and 2018. Table 2 shows the detailed racial distribution of disease-specific clinical trials. Whites made up a majority of clinical trial population (above 70%) across all diseases except in SLE (57.62%). Asians were the 2nd most common race represented except in gout. When compared with the 2020 US census by the proportion of race, black subjects were underrepresented in RA, SLE, SSc, and SpA clinical trials and overrepresented in gout.
Native Hawaiian or Other Pacific Islanders were least represented (< 1%).
Conclusion: Black study subjects continued to be underrepresented in rheumatology clinical trials except for gout.
In the era of evidence-based medicine, adequate racial representation in clinical trials is prudent. It is crucial that the results of these “practice-changing” trials are generalizable.
NIH Revitalization Act passed in 1993 emphasized women and minorities’ inclusion in NIH-sponsored clinical trials.[1] Practically, many pivotal trials not funded by NIH were exempt. Racial minorities continued to be underrepresented in rheumatology clinical trials. Meaningful actions at the research regulatory level, or point of trial conception, are needed to foster inclusivity in clinical trials.
References:
NIH Revitalization Act. Subtitle B: §131-133; 1993.
To cite this abstract in AMA style:
Khandwala P, Hussain A, Tan I. Racial Disparities in Rheumatology Clinical Trials [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/racial-disparities-in-rheumatology-clinical-trials/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/racial-disparities-in-rheumatology-clinical-trials/