Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Biologic and targeted synthetic DMARDs can substantially improve the quality of life for Medicare beneficiaries with rheumatoid arthritis (RA). However, racial and ethnic disparities in their use, particularly those covered by Part B, have not been well studied in this population. Medicare defines any drug costing over $670 per patient per month as a “specialty” drug, which encompasses all biologic and targeted synthetic DMARDs. We examined racial and ethnic differences in 12-month use of any DMARD, specialty DMARDs, and the Part B-covered subset in a large cohort of Medicare enrollees with late-onset RA.
Methods: We used 20% Sample Medicare claims from 2016-2020 to establish an incident cohort of late-onset RA, defined as ≥2 outpatient ICD-10 RA codes 7-365 days apart following a 12-month washout. Eligible beneficiaries were ≥65 years old, continuously enrolled in Medicare Parts A/B/D, and had no other indication for DMARD use. Outcomes included receipt of (1) any DMARD, (2) specialty DMARDs, and (3) Part B-covered subset within 12 months of case identification. The main regressor was race and ethnicity (non‑Hispanic White reference). Covariates included age, sex, and low‑income subsidy status (LIS). We performed multivariable logistic regressions for each outcome, incorporating calendar‑month and calendar‑year fixed effects, and reporting adjusted ORs (aORs) with 95% CIs.
Results: Among 39,706 beneficiaries with incident late-onset RA (Figure), 14,190 (35.7%) received any DMARD, 1,906 (4.8%) a specialty DMARD, and 1,363 (3.4%) a Part B–covered specialty DMARD within 12 months (Table 1). The most common first DMARDs included:- Any DMARD: methotrexate (6,154), hydroxychloroquine (5,229), and leflunomide (850); – Specialty: abatacept (278; both formulations), certolizumab (274; both formulations), and infliximab (259); – Part B-covered subset: certolizumab (270), infliximab (255), and abatacept (253).In multivariable models (Table 2) Black patients (aOR: 0.92) had significantly lower odds of receiving any DMARD than non-Hispanic White patients. Race and ethnicity were not associated with differential receipt of specialty DMARDs. However, in the Part B-covered subset, Black (aOR 0.78), Hispanic (aOR 0.75), and Other (aOR 0.63) patients had significantly lower odds of receiving these agents compared to non-Hispanic White patients.Also, LIS was associated with 14%, 15%, and 67% lower odds of any DMARD use, specialty DMARD use, and the Part B-covered subset, respectively. Moreover, each additional year of age was associated with 4%, 5%, and 6% lower odds of any DMARD use, specialty DMARD use, and Part B therapy use, respectively (with mild nonlinearity modeled via age²). Furthermore, female sex was associated with 13% lower odds of specialty DMARD use.
Conclusion: Part B-covered DMARDs exhibited the most pronounced disparities: Black, Hispanic, and Other non-White patients were 22-37% less likely to receive these therapies. This gap is particularly concerning because, despite the inconvenience of office or infusion-center administration, Part B agents carry lower coinsurance than Part D’s specialty tier, representing a key cost-saving opportunity that non-White beneficiaries may be missing.
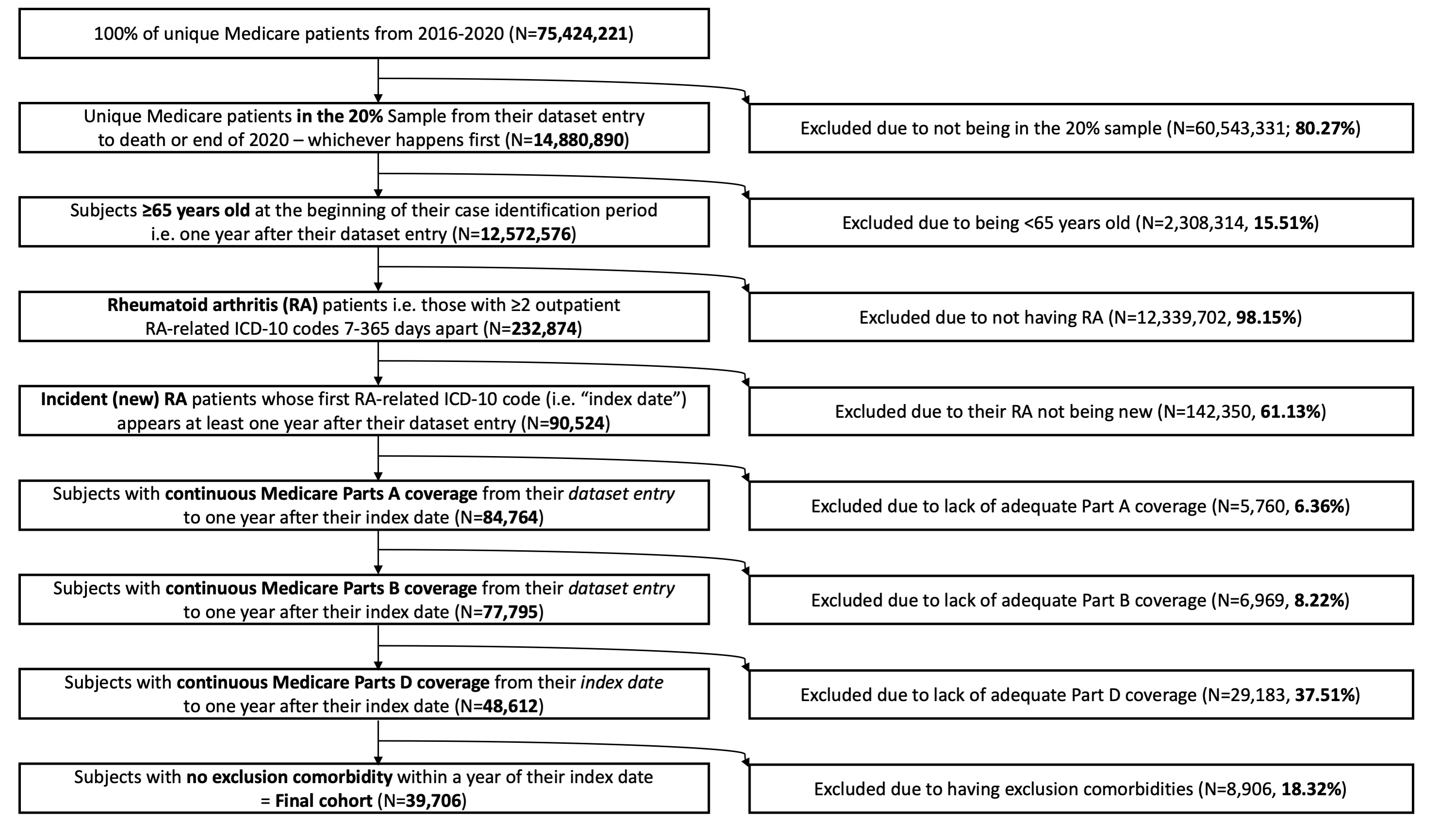 Figure: Flow diagram depicting the establishment of a cohort with incident late-onset RA among Medicare beneficiaries (2016-2020).
Figure: Flow diagram depicting the establishment of a cohort with incident late-onset RA among Medicare beneficiaries (2016-2020).
.jpg) Table 1: Baseline characteristics of the included patients, overall and by receipt of first DMARD (any), first specialty DMARD, and first Part B-covered specialty DMARD (i.e. office- or infusion-center–administered) within 12 months of case identification.
Table 1: Baseline characteristics of the included patients, overall and by receipt of first DMARD (any), first specialty DMARD, and first Part B-covered specialty DMARD (i.e. office- or infusion-center–administered) within 12 months of case identification.
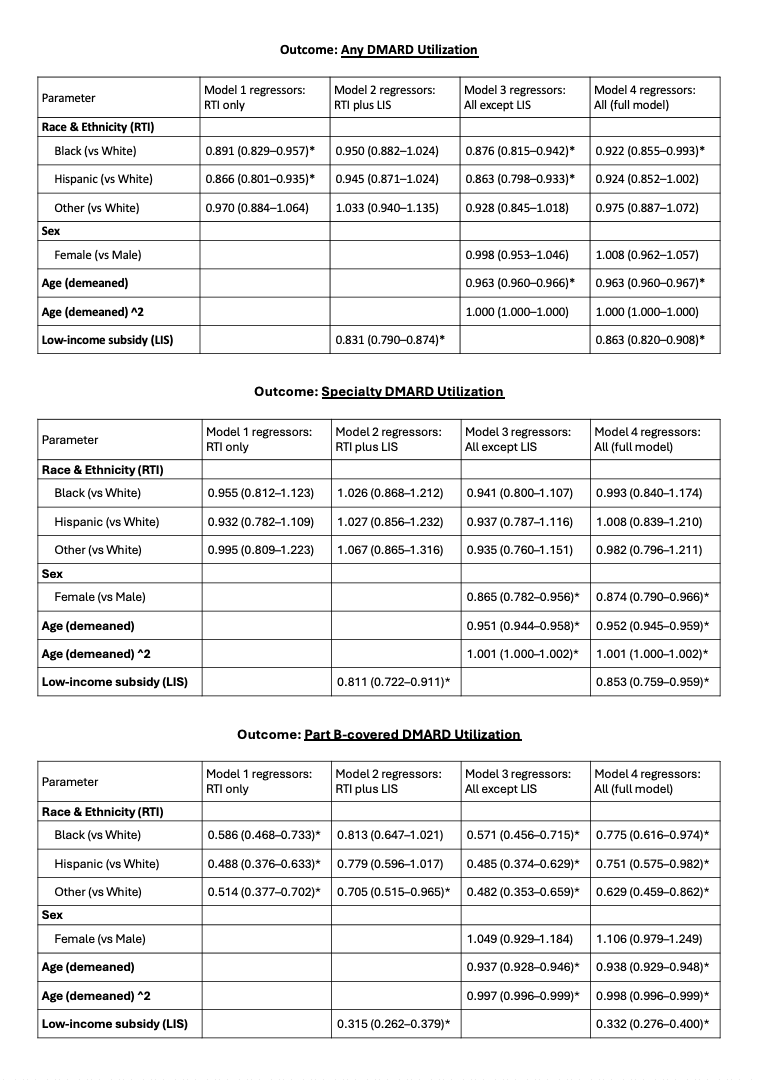 Table 2. Adjusted odds ratios (95% CI) from multivariable logistic regression models examining the association of race and ethnicity, sex, age, and low‐income subsidy status (LIS: proxy for socioeconomic status) with (A) any DMARD use, (B) specialty DMARD use, and (C) Part B-covered specialty DMARD use within 12 months of case identification. Four model specifications are shown per outcome: Model 1 (race and ethnicity only), Model 2 (+ LIS), Model 3 (all covariates except LIS), and Model 4 (full model). Significant findings (p < 0.05) are indicated by an asterisk.
Table 2. Adjusted odds ratios (95% CI) from multivariable logistic regression models examining the association of race and ethnicity, sex, age, and low‐income subsidy status (LIS: proxy for socioeconomic status) with (A) any DMARD use, (B) specialty DMARD use, and (C) Part B-covered specialty DMARD use within 12 months of case identification. Four model specifications are shown per outcome: Model 1 (race and ethnicity only), Model 2 (+ LIS), Model 3 (all covariates except LIS), and Model 4 (full model). Significant findings (p < 0.05) are indicated by an asterisk.
To cite this abstract in AMA style:
ara a, FitzGerald J, Ettner S. Racial and Ethnic Disparities in DMARD Use and in Medicare Part B-Covered Options Among Medicare Beneficiaries with Late-Onset Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/racial-and-ethnic-disparities-in-dmard-use-and-in-medicare-part-b-covered-options-among-medicare-beneficiaries-with-late-onset-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/racial-and-ethnic-disparities-in-dmard-use-and-in-medicare-part-b-covered-options-among-medicare-beneficiaries-with-late-onset-rheumatoid-arthritis/
