Session Information
Date: Monday, November 18, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Gout management guidelines endorse treat-to-target (T2T) urate lowering therapy (ULT) to achieve and maintain serum urate (sUA) < 6.0 mg/dl. While T2T ULT interventions have demonstrated improved outcomes as early as 1- to 2-years compared to usual care, the durability of long-term ULT following T2T implementation has not been extensively studied. Examining follow up data from a randomized controlled trial comparing T2T management with allopurinol and febuxostat in gout, we evaluated the frequency and determinants of ULT persistence up to 2 years post-study completion.
Methods: This analysis examined participants completing the 72-week STOP Gout trial (n=740) conducted in the Veterans Affairs (VA) healthcare system, excluding non-veteran participants (n=46), deaths (n=35), and those lost to VA follow-up (n=21) before accumulating 2 years of post-study observation. Participants were followed passively using administrative data with persistence defined as any ULT dispensing (allopurinol, febuxostat, pegloticase, or probenecid) overlapping with the 2-year post-study time point. Associations of participant factors with ULT persistence were examined using univariable and multivariable logistic regression. Potential determinants of ULT persistence examined included participant demographics, comorbidity, health-related quality of life, healthcare utilization, post-study rheumatology care, and gout-related factors.
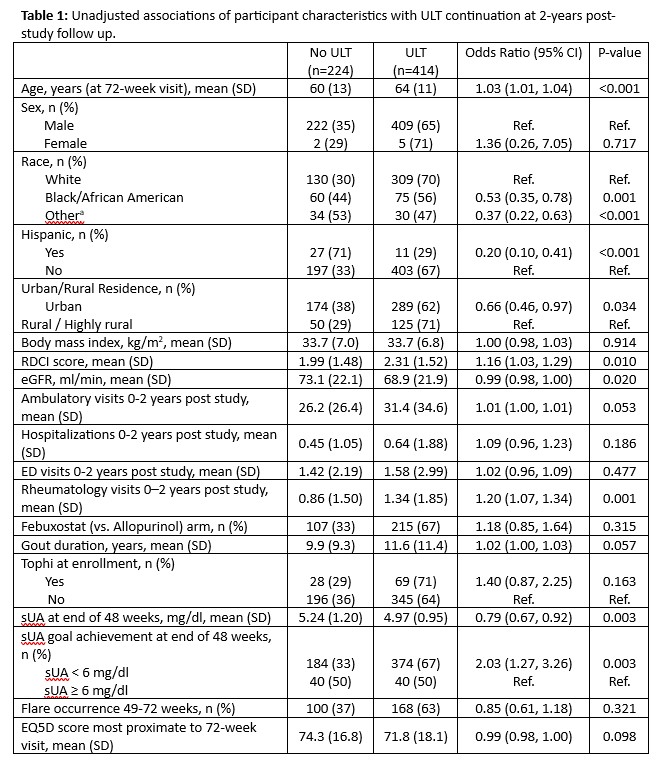
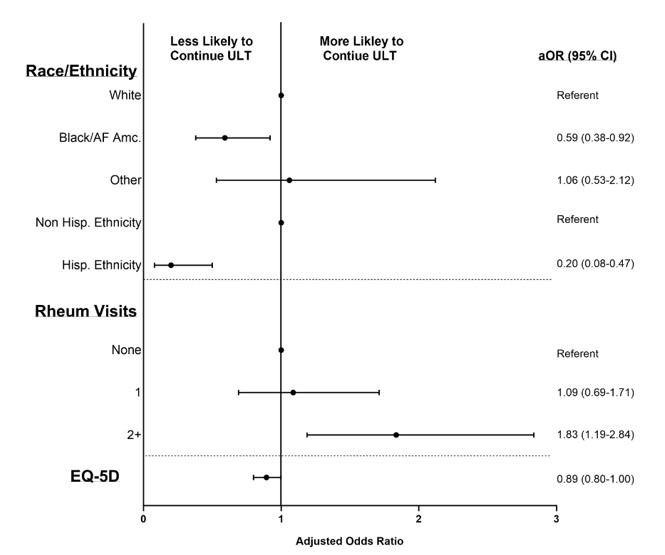
Results: STOP Gout completers included in this analysis (n=638) were older (mean age 63 years), 99% male, and 31% reported non-white race. At 2 years post-study follow-up, 65% of participants were dispensed ULT. ULT discontinuation increasingly occurred throughout post-study observation (4% of discontinuations between 0-3 months; 11% 4-6 months; 14% 7-12 months and 59% during year 2). Unadjusted associations of participant factors with ULT persistence post-study are shown in Table 1. After accounting for covariates, the occurrence of 2 or more rheumatology visits post-study was associated with better ULT persistence (aOR 1.83; 95% CI 1.19-2.84) (Figure 1). Lower ULT persistence was observed among individuals reporting Black or African American race (vs. white), Hispanic ethnicity (vs. non-Hispanic) and reduced quality of life at the beginning of post-study follow-up. Achievement of sUA < 6 mg/dl at 48 weeks during the STOP Gout study demonstrated a borderline association with greater long-term persistence (aOR 1.66; 0.98-2.82). Using laboratory values most proximal to 2-years post-study, 65% were at sUA goal.
Conclusion: Though interventions implementing T2T ULT have demonstrated efficacy, this study suggests that such interventions may have limited durability following transitions back to real-world gout management. The issue of limited treatment durability appears to be compounded among underrepresented patient populations and improved in the context of rheumatology subspecialty care. These results suggest the need for interventions specifically designed to facilitate continued treatment maintenance following initial successful implementation of T2T ULT in gout management.
Abbreviations: ULT, urate-lowering therapy; RDCI, Rheumatic Disease Comorbidity Index; eGFR, estimated glomerular filtration rate; sUA, serum urate; EQ5D, EuroQol 5-dimension
Variables with p-value < 0.05 shown; other covariates in multivariable model (all with p-values <0.2 in univariable models but p >0.05 in multivariable model) include: age (OR 1.02; 95% CI 1.00, 1.03), RDCI score (OR 1.03; 95% CI 0.90, 1.18), urban vs. rural residence (OR 0.79; 95% CI 0.52, 1.20), eGFR (OR 1.00; 95% CI 0.99, 1.01), number of ambulatory visits 0_2 years post study (OR 1.26; 95% CI 0.82, 1.95 for 16_30 visits and OR 1.22 95% CI 0.73, 2.04 for ≥31 visits vs. <16 visits), any inpatient hospitalization 0_2 years post study (OR 0.81; 95% CI 0.51, 1.27), tophi at enrollment (OR 1.06; 95% CI 0.63, 1.79), gout duration at 72 weeks (OR 1.01; 95% CI 1.00, 1.03), sUA <6 mg/dl at 48 weeks (OR 1.66; 95% CI 0.98, 2.82)
Abbreviations: aOR, adjusted odds ratio; AF Amc., African American; ULT, urate-lowering therapy; Hisp., Hispanic; Rheum, rheumatology; EQ-5D, EuroQoL 5-dimension; RDCI, Rheumatic Disease Comorbidity Index; eGFR, estimated glomerular filtration rate
To cite this abstract in AMA style:
Kohn S, Sayles H, Helget L, England B, Roul P, Qu J, Newcomb J, Kramer B, Duryee M, Davis-Karim A, Brophy M, Ferguson R, Pillinger M, Neogi T, Palevsky P, Wu H, O'Dell J, Mikuls T. Race, Ethnicity, and Rheumatology Care Predict Long-term Urate-Lowering Treatment Persistence Following a Treat-to-Target Intervention in a Large, Multicenter Randomized Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/race-ethnicity-and-rheumatology-care-predict-long-term-urate-lowering-treatment-persistence-following-a-treat-to-target-intervention-in-a-large-multicenter-randomized-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/race-ethnicity-and-rheumatology-care-predict-long-term-urate-lowering-treatment-persistence-following-a-treat-to-target-intervention-in-a-large-multicenter-randomized-trial/