Session Information
Date: Monday, November 11, 2019
Title: 4M115: Pain Mechanisms – Basic & Clinical Science (1878–1883)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: In recent years, diverse compounds for intra-articular (IA) administration were brought into the market with a subsequent significant and heterogeneous literature production.
Understanding the efficacy of any drug to be delivered intra-articularly implies bearing in mind the placebo (PBO) effect of any medication that is placed where the pain is elicited, especially in the first weeks after the procedure. To date, most of the studies analyzing this effect were not focused specifically on the route of administration but on the treatment being administered. Thus, we aimed at evaluating the size of the PBO effect after IA injections.
Methods: For the upcoming EULAR recommendations/points to consider for IA therapies, an overview of systematic reviews (SR) was conducted, including SR of randomized-controlled trials (RCT) of IA therapies comparing an active treatment vs an IA control. We selected all SR in which the control arm was a saline solution. After assessing the risk of bias with the AMSTAR-2 tool, all SR showing high-confidence results were selected and the studies included in them, re-analyzed.
The analysis included data on the change in pain from the PBO arms, measured on continuous variables (different scales) from baseline to 3-6 and 12-16 weeks after the IA procedure. The standardized mean differences (SMD) from baseline were calculated as the ratio between the size of the intervention effect in each study and the variability observed in that study. A meta-analysis was performed using an inverse-variance random-effects model in Review Manager 5.3. The overall effect sizes obtained refer to versions of the SMD, which corresponds to the Hedges’ (adjusted) g. e.g. a “g” of 1 indicates the two groups being compared differ by 1 standard deviation and so on.
Results: Two SR comprising 50 RCT were included, 44 had to be excluded: 26 due to not having PBO arms or not showing its data, 6 due to not measuring the target outcome or at the specified time-points, in 5 we could not obtain the target data, and 7 used a PBO different than saline.
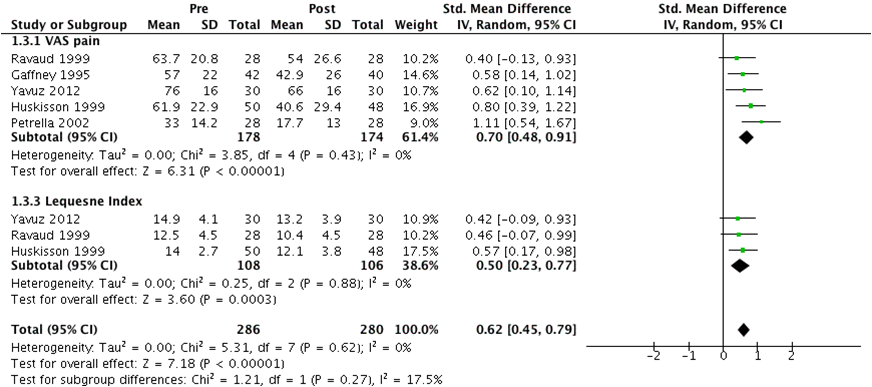
Pain, measured by visual analog scale (VAS) and Lequesne index, was retrieved from 6 RCT. The effect of PBO IA administration from baseline to 3-6 weeks was pooled from 6 studies on VAS pain and 3 on Lequesne index; SMD [95%CI] was 0,74 [0.47-1.00]. As one study had too large an effect, we performed a sensitivity analysis excluding it. This resulted in a notable reduction of overall heterogeneity both measured by I2 and Tau2 with a resultant SMD = 0.62 [0.45-0.79] (Figure 1).
The overall PBO effect at 12-16 weeks, measured by pooling data from 3 studies for VAS pain and for Lequesne, showed an SMD = 0.33 [0.14-0.52] (Figure 2).
Using the interpretation suggested by Cohen1, our results would confirm a moderate to large effect of IA saline (PBO) at 3-6 weeks with a subsequent reduction to a small but persistent effect at 12-16 weeks. Therefore, IA PBO may have a clinically detectable and lasting effect.
Conclusion: Based on these findings we suggest that PBO effect should be taken into account not only when analyzing the efficacy of IA therapies in RCT but also in clinical practice, where this effect could be maximized, as well.
References:
- Cohen J. Statistical Power Analysis in the Behavioural Sciences. 1988.
To cite this abstract in AMA style:
Rodriguez-Garcia S, Castellanos-Moreira R, Uson-Jaeger J, NAREDO E, Carmona L. Quantifying the Placebo Effect After Intra-Articular Injections: Implications for Trials and Practice [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/quantifying-the-placebo-effect-after-intra-articular-injections-implications-for-trials-and-practice/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/quantifying-the-placebo-effect-after-intra-articular-injections-implications-for-trials-and-practice/