Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Pyruvate kinase M2 (PKM2), one of glycolytic enzymes, is an emerging molecule that plays significant roles in tumor growth as well as inflammatory process. However, the role of PKM2 in the RA pathogenesis has not been well evaluated. The aim of this study is to investigate the expression of PKM2 in RA patients and to find their clinical implications. Also, we tried to determine the role of PKM2 in the biologic processes of RA fibroblast-like synoviocytes (FLSs) and osteoclast in vitro.
Methods: To determine expression and localization of PKM2 in RA synovial tissue, immunohistochemistry and double IF was performed. The levels of PKM2 in SF and plasma were measured by ELISA and their relations with clinical variables including modified Sharp/van der Heijde (SHS) scores and circulating levels of TNF-α, IL-6, and VEGF were analyzed. In RA FLSs and THP-1 cell lines stimulated with LPS, TNF-α, or IL-6, the PKM2 levels in the conditioned media were also investigated. The effect of recombinant PKM2 on osteoclast differentiation was examined in Raw264.7 cell line. Additionally, the impact of PKM2 down-regulation on migration and proliferation in RA-FLSs were determined using small-interfering RNA.
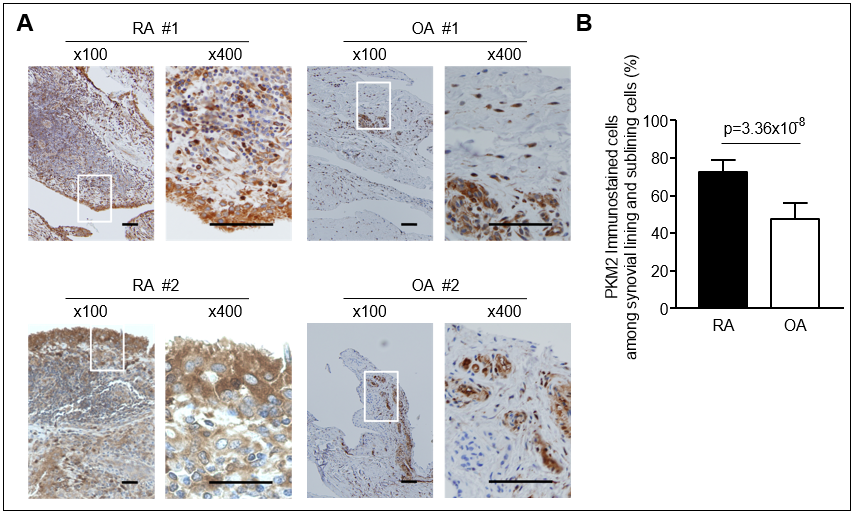
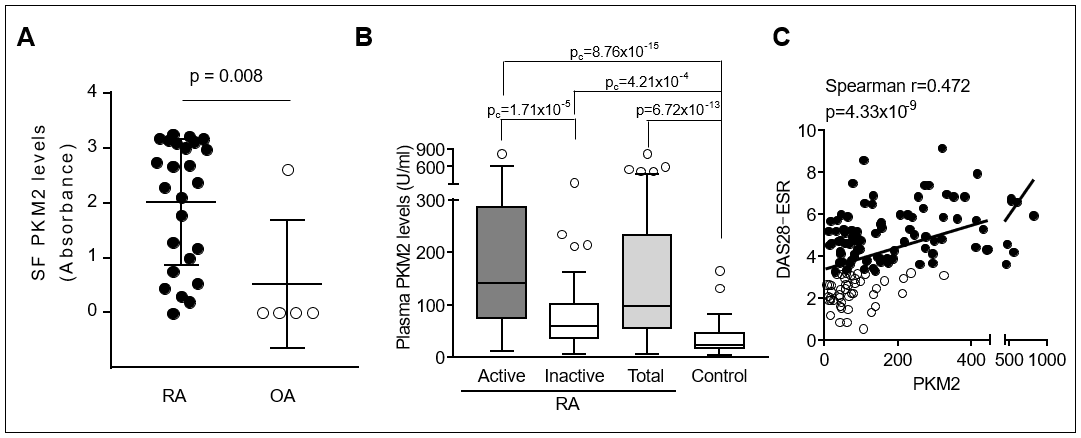
Results: In the synovial tissues of RA patients, PKM2 was more immunostained than in OA synovium and mainly expressed in the fibroblasts. The levels of SF PKM2 in RA patients were significantly higher than those in OA patients and correlated with SF inflammatory cell counts. Compared with healthy controls, the plasma PKM2 levels were significantly elevated in RA patients and correlated with DAS28. The circulating levels of PKM2 were positively correlated with IL-6 and VEGF levels, but not with TNF-α. Elderly onset RA patients with high PKM2 levels (≥ 90th percentile of the controls) were more likely to have radiographic progression (Δ SHS ≥1 unit/year) than those with low PKM2 levels. In early RA (disease duration ≤12 months) subgroup, plasma PKM2 levels were independent predictors for subsequent progression of radiographic damage. In vitro, PKM2 release was enhanced in stimulated macrophages, but not in stimulated RA-FLSs. The number of tartrate resistant acid phosphatase-positive multinuclear cells was dose-dependently increased by recombinant PKM2 in Raw264.7 cell, even without RANK ligand. PKM2 knockdown by siRNA transfection significantly decreased TNF-α induced migration and proliferation of RA-FLSs.
Conclusion: PKM2 was upregulated in RA synovium and the elevated PKM2 levels in plasma and SF were associated with inflammatory burden as well as radiographic progression. Intracellular PKM2 could be involved in the migration and proliferation of RA-FLSs, whereas extracellular PKM2 released from activated macrophages could promote osteoclastogenesis. These findings suggest that PKM2 might be a novel regulator in the pathogenesis of RA.
To cite this abstract in AMA style:
Han D, Choi Y, Ha Y, Park E, Kang E, Song Y, Lee Y. Pyruvate Kinase M2 May Contribute to the Inflammation and Joint Destruction in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/pyruvate-kinase-m2-may-contribute-to-the-inflammation-and-joint-destruction-in-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pyruvate-kinase-m2-may-contribute-to-the-inflammation-and-joint-destruction-in-rheumatoid-arthritis/