Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Paradoxical psoriasis has been increasingly reported in adults after exposure to tumor necrosis factor inhibitors (TNFi). Systematic studies in the pediatric population are lacking. We aimed to investigate the relationship between TNFi therapy and the onset of new psoriasis in children with juvenile idiopathic arthritis (JIA) using Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry data.
Methods: De-identified data were obtained from CARRA registry for patients enrolled in CARRA Registry with a diagnosis of JIA from June 30, 2015 to January 1, 2020 with follow up data available. Patients with Inflammatory Bowel Disease or psoriasis documented on or prior to JIA diagnosis date were excluded, as were patients with incomplete data regarding medication start/stop dates. New onset psoriasis was defined by first recorded instance of psoriasis following JIA diagnosis. Exposure time to TNFi was defined as: 1. Ever Exposure: any time observed after the patient’s first exposure to a TNFi until censoring (psoriasis developed, or most recent visit date if continuing TNFi, or 60 days after the discontinuation of the TNFi, whichever was sooner); or 2. Total Exposure: any time observed while actively on TNFi, until censoring; or 3. First Exposure Only: any time observed while actively on the first TNFi, until censoring. Baseline characteristics were analyzed with descriptive statistics. Hazard ratios were calculated between exposed and unexposed groups adjusted for methotrexate exposure, sex, race, family history of psoriasis and initial JIA category.
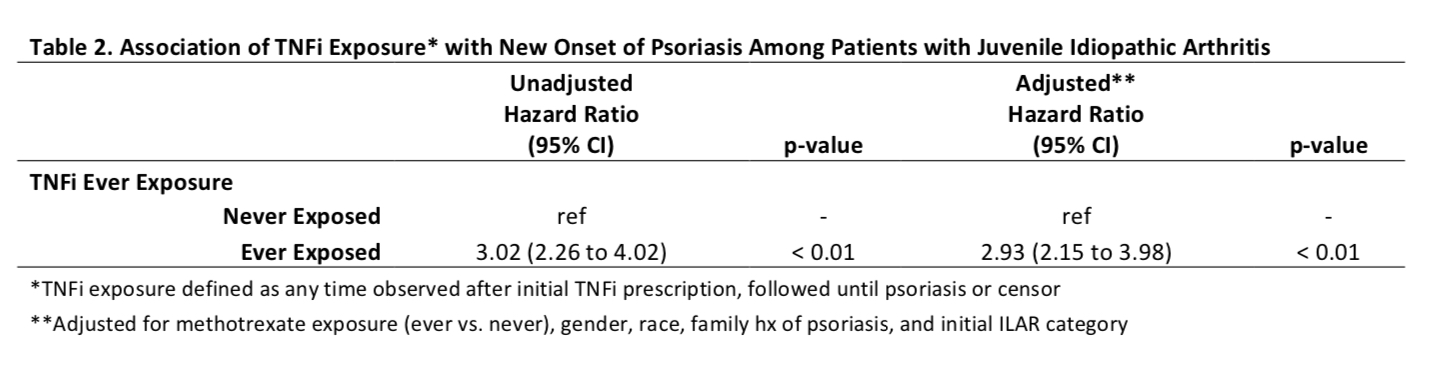
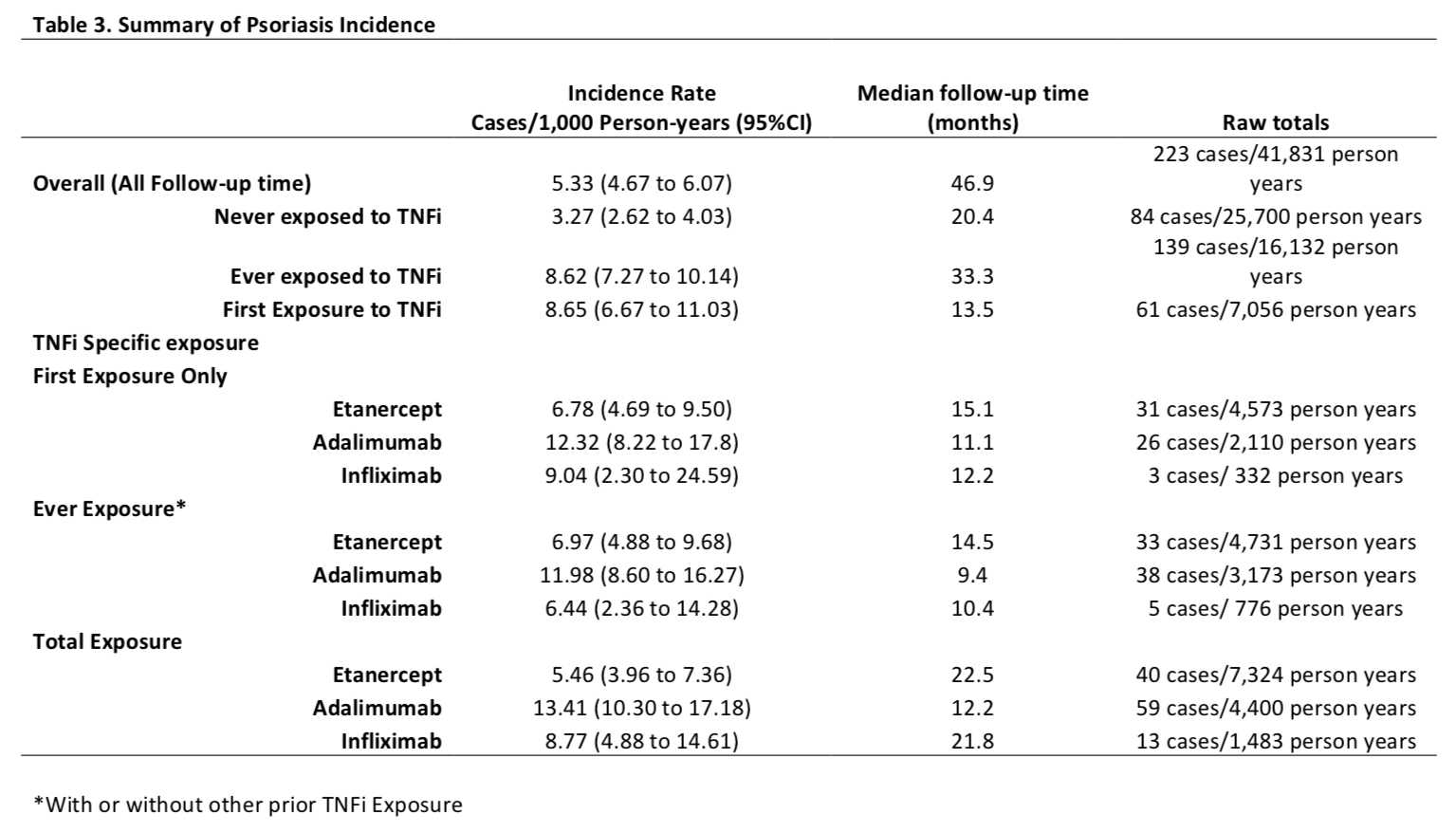
Results: A total of 8,222 patients were included with a median follow up of 5.3 years. Over half of the patients were prescribed TNFi (n=4,435, 54%; Table 1). The hazard ratio of new onset of psoriasis after Ever Exposure to TNFi was 3.02 (CI 2.26 to 4.02, unadjusted) and 2.93 (2.15 to 3.98, adjusted) (p< 0.01) (Table 2). The incidence rate of psoriasis was the highest in children who received adalimumab in Ever Exposure, Total Exposure and First Exposure Only calculations (Table 3). A subanalysis of patients with psoriatic arthritis showed that the risk of new onset TNFi-associated psoriasis was increased but the increased risk was lower than observed in the other categories combined. The adjusted hazard ratios (95% CI) of psoriasis after TNFi exposure was 1.68 (1.11 to 2.54, adjusted, p=0.01) in psoriatic JIA and 5.60 (3.47 to 9.05, adjusted, p< 0.01) in non-psoriatic JIA.
Conclusion: In a large prospective JIA patient registry, we observed a nearly 3 fold increased risk of psoriasis after TNFi exposure. Increasing awareness of this unwanted side effect in pediatric community is important to ensure timely diagnosis and treatment. Authors include the CARRA Registry Investigators
To cite this abstract in AMA style:
Zhao y, Sullivan E, Son M, Beukelman T. Psoriasis Rate Is Increased by the Exposure to TNF Inhibition in Children with JIA [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/psoriasis-rate-is-increased-by-the-exposure-to-tnf-inhibition-in-children-with-jia/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/psoriasis-rate-is-increased-by-the-exposure-to-tnf-inhibition-in-children-with-jia/