Session Information
Date: Monday, November 18, 2024
Title: SpA Including PsA – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The Psoriatic Arthritis (PsA) Impact of Disease questionnaire (PsAID12) score (range 0-10) allows for the assessment of patient-important symptoms and life impact in PsA. Thresholds defining different levels of disease impact for the PsAID12 have been recently published in the setting of randomised controlled trials (RCT) [1], while previously different thresholds developed in an observational (OBS) population were proposed [2]. However, thresholds differ based on the setting used for their development, and no consensus exists concerning the preferred thresholds. The objectives of this study were: a) to compare the percentage of patients captured at the different impact status according to the RCT and OBS thresholds in the ReFlaP observational cohort; b) to evaluate the agreement between these thresholds for classifying patients with each status; c) to evaluate their sensitivity and specificity against the DAPSA (Disease Activity index in Psoriatic Arthritis) status used as the external standard.
Methods: The OBS and RCT sets of thresholds for the PsAID12 were applied to patients in the ReFlaP observational study (NCT03119805) at the baseline visit [2]. Patients were divided into remission, low, moderate, and high disease impact according to the RCT and OBS thresholds. Weighted kappa was used to evaluate the agreement across status. Finally, the sensitivity and specificity of these thresholds (both RCT and OBS) were evaluated using the DAPSA status as the external standard (which was also used for defining the thresholds).
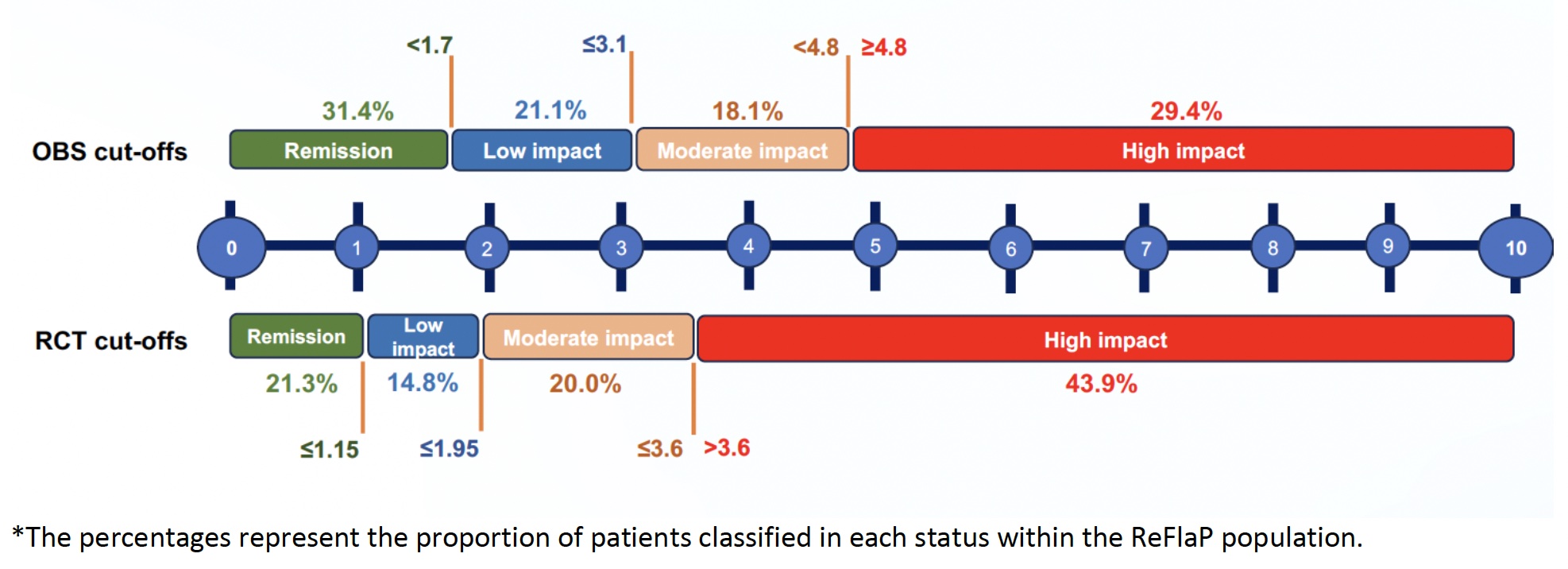
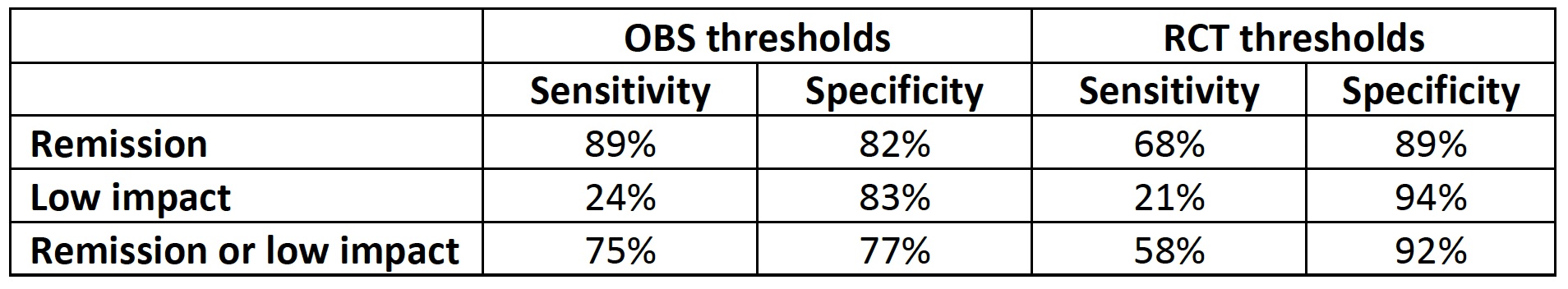
Results: A total of 465 patients from the ReFlaP cohort were evaluated (50.3% males, mean age 53.4 years). The mean (SD) PsAID12 total score at baseline was 3.4 (2.5). Thresholds for remission, low, moderate, and high impact identified in the RCTs were more stringent compared to those identified in the OBS setting (Figure 1). A total of 21.1% and 31.4% were classified in remission according to the RCT and OBS thresholds, respectively, while 14.8% and 21.1% were classified as low impact. Substantial agreement (weighted kappa: 0.703) across status using the RCT and OBS thresholds was observed. Finally, OBS thresholds showed higher sensitivity than the RCT thresholds for remission (i.e., the ability of the thresholds to correctly classify patients in remission), while the RCT thresholds showed better specificity (Table 1).
Conclusion: Thresholds defined for PsAID12 in a RCT did not fully overlap with those proposed in an OBS setting but showed substantial agreement. Thresholds developed in RCTs were more stringent than the OBS thresholds (potentially reflecting differences in patient characteristics and status) and had higher specificity against DAPSA status. However, OBS thresholds showed better sensitivity for classifying patients in remission. A wider consensus is needed to stablish the official cut-offs.
To cite this abstract in AMA style:
López Medina C, Orbai A, Lubrano E, Coates L, Kiltz U, Leung Y, PALOMINOS P, Cañete J, Scrivo R, Balanescu A, Dernis E, Meisalu S, Ruyssen-Witrand A, Soubrier M, Aydin S, Eder L, Kalyoncu U, Smolen J, de Wit M, Gossec L. PsAID12 Thresholds Defining Disease Impact Severity in Psoriatic Arthritis Developed in an Observational Setting Showed Higher Sensitivity but Lower Specificity Than Those Developed in a Randomized Controlled Trial Setting [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/psaid12-thresholds-defining-disease-impact-severity-in-psoriatic-arthritis-developed-in-an-observational-setting-showed-higher-sensitivity-but-lower-specificity-than-those-developed-in-a-randomized-co/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/psaid12-thresholds-defining-disease-impact-severity-in-psoriatic-arthritis-developed-in-an-observational-setting-showed-higher-sensitivity-but-lower-specificity-than-those-developed-in-a-randomized-co/