Session Information
Date: Tuesday, November 14, 2023
Title: Abstracts: Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Patients with inflammatory rheumatic and musculoskeletal diseases (iRMDs) are at increased risk of osteoporosis and fragility fractures. For this population, whether proton pump inhibitor (PPI) intake contributes to that risk has not yet been definitively answered. Prior studies have yielded conflicting results, did not account for over-the-counter use of PPI, and major confounders were unmeasured. In addition, bone microarchitecture as a potential mediator of fracture risk has not been studied. We studied the effect of regular PPI intake on bone mineral density (BMD) and microarchitecture in patients with iRMDs.
Methods: Cross-sectional baseline data from the single center Rh-GIOP cohort were used. Briefly, patients with iRMDs were prospectively enrolled and assessed with DXA scans, laboratory testing, and bone-health-related questionnaires since 2015. Regular PPI and glucocorticoid (GC) use were ascertained by both chart review and patient self-report. Three co-primary outcomes (all reported as T-scores) were defined: BMD of the left femoral neck and the lumbar spine, and the trabecular bone score (TBS). The latter is a measure correlating with lumbar vertebrae’s microarchitecture and is measured in a subset. Inverse probability of treatment weighting adjusted for the following confounders: age, sex, body mass index, iRMD type, C-reactive protein, current and cumulative GC dose, NSAID use, smoking, alcohol consumption, functional status (Health Assessment Questionnaire), disease duration, bisphosphonate use, chronic kidney disease stage, presence of diabetes mellitus, and frequency of exercise. We investigated whether dose-response relationships were present (“high dose” of >20mg/d vs. “low dose” of ≤20mg/d pantoprazole equivalent) and conducted an additional analysis with an interaction term for GC use. All analyses were based on linear regression models. Multiple imputation with 100 imputations was used to account for missing data (~4%). A detailed prespecified statistical analysis plan with a gatekeeping procedure for statistical testing was followed.
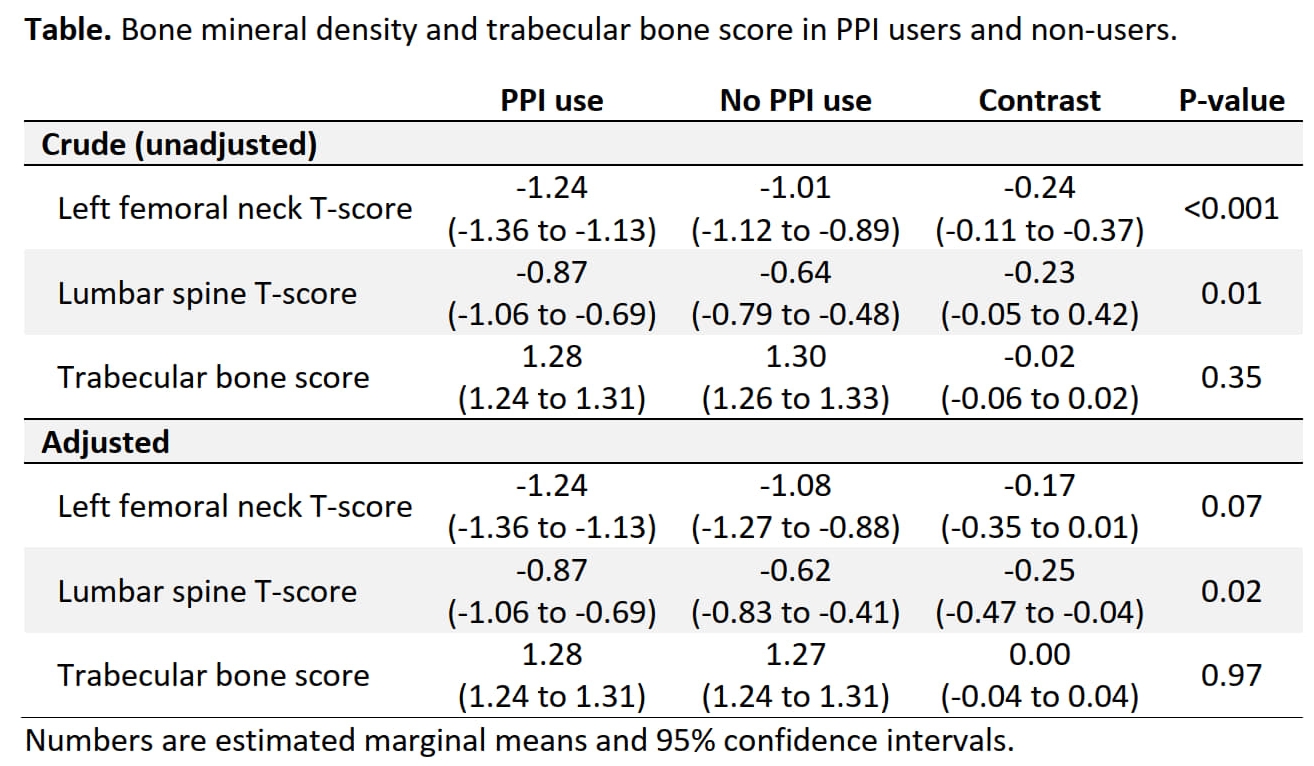
Results: 1,495 patients (75.3% women; mean age 62.6 ± 13.1 years; 49.2% with regular PPI use [of those: 63.1% high dose]) were included. Most patients had a diagnosis of rheumatoid arthritis (37.5%), followed by 25.3% connective tissue diseases, 16% vasculitides, 14.2% spondyloarthropathies, and 7% others. 63.8% used GCs (median dose 5mg/d). In both adjusted and unadjusted analyses, PPI users had lower BMD at both the left femoral neck and the lumbar spine (Table). Interestingly, differences between PPI users and non-users were only present in the subset of patients concurrently using GCs (data not shown). There was no statistically significant difference in BMD when comparing high and low dose PPI users (all P ≥ 0.52). TBS (n = 389) was similar in PPI users and non-users (Table).
Conclusion: Loss of BMD (seen at both lumbar spine and left femoral neck) rather than impairment of bone microarchitecture seems to be driving the increased fracture risk seen with PPI use in patients with iRMDs. The negative association between PPI use and BMD appears to be dependent on concurrent GC use.
To cite this abstract in AMA style:
Palmowski A, Schmajuk G, Yazdany J, Katz P, Li J, Stovall R, Kersey E, Nielsen S, Christensen R, Bliddal H, Boyadzhieva Z, Schneider U, Alexander T, Muche B, Hermann S, Wiebe E, Buttgereit F. Proton Pump Inhibitor Use Is Associated with Impaired Bone Mineral Density but Not Bone Microarchitecture in Patients with Inflammatory Rheumatic and Musculoskeletal Diseases Taking Glucocorticoids [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/proton-pump-inhibitor-use-is-associated-with-impaired-bone-mineral-density-but-not-bone-microarchitecture-in-patients-with-inflammatory-rheumatic-and-musculoskeletal-diseases-taking-glucocorticoids/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/proton-pump-inhibitor-use-is-associated-with-impaired-bone-mineral-density-but-not-bone-microarchitecture-in-patients-with-inflammatory-rheumatic-and-musculoskeletal-diseases-taking-glucocorticoids/