Session Information
Date: Friday, November 6, 2020
Session Type: Plenary Session
Session Time: 11:30AM-1:00PM
Background/Purpose: Patients with coronavirus disease 19 (COVID-19) are at high risk for thrombosis of arteries and veins. At the same time, COVID-19 lung histopathology has revealed fibrin-based occlusion of small vessels. Antiphospholipid syndrome (APS) is an acquired thrombophilia in which patients develop pathogenic autoantibodies (aPL) targeting phospholipids and phospholipid-binding proteins. Small case series have recently detected aPL in patients with COVID-19. Here, we endeavored to comprehensively measure aPL in a large cohort of patients hospitalized with COVID-19. We also asked whether purified IgG fractions from these patients had prothrombotic properties in vitro and in vivo.
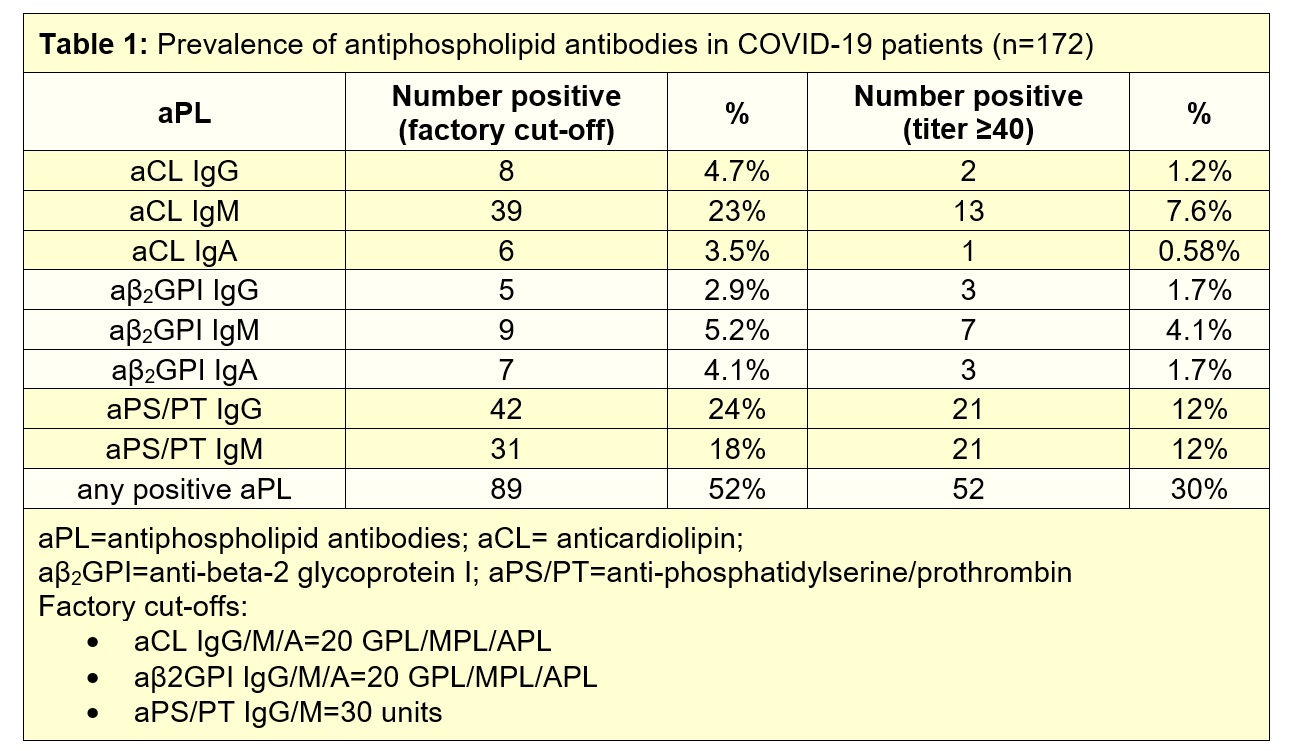
Methods: Sera from 172 patients hospitalized with COVID-19 were evaluated for eight different types of aPL: anticardiolipin IgG/IgM/IgA, anti-beta-2 glycoprotein I IgG/IgM/IgA, and anti- phosphatidylserine/prothrombin (PS/PT) IgG/IgM. IgG fractions were purified from COVID-19 patients with various aPL profiles. The prothrombotic potential of the purified IgG fractions were evaluated in neutrophil extracellular trap (NET) assays and in two mouse models of inferior vena cava thrombosis.
Results: We detected anticardiolipin IgM antibodies in 23% of COVID-19 patients, anti-PS/PT IgG in 24%, and anti-PS/PT IgM in 18%. Any aPL was present in 52% of patients based upon the manufacturer’s threshold and in 30% using a more stringent cutoff (≥40 units) (Table 1). High aPL levels tended to track with more severe COVID-19. For example, anti-PS/PT IgM levels were significantly correlated with neutrophil activation (calprotectin, r=0.23, p=0.002), circulating NETs (myeloperoxidase/MPO-DNA complexes, r=0.23, p=0.003), and more severe respiratory disease (SpO2/FiO2, r=-0.16, p=0.03). Similar correlations were also seen for anticardiolipin IgM (calprotectin r=0.28, p=0.0002; MPO-DNA r=0.25, p=0.001; SpO2/FiO2 r=-0.19, p=0.01). Furthermore, positive testing for any aPL predicted impaired kidney function as defined by estimated glomerular filtration rate (p=0.03). Purified IgG fractions from aPL-positive COVID-19 patients (either anti-β2GPI IgG or anti-PS/PT IgG) promoted NET release from control neutrophils, similar to IgG isolated from individuals with established APS. Furthermore, injection into mice of COVID-19 IgG fractions (isolated from four different patients with high levels of anti-PS/PT IgG) more than doubled thrombus extension and accretion in two separate models of inferior vena cava thrombosis. Administration of the COVID-19 IgG also significantly increased circulating NET remnants in mice (p=0.0004), similar to IgG from patients with catastrophic antiphospholipid syndrome (p=0.001).
Conclusion: These data demonstrate that a significant percentage of patients with COVID-19 become at least transiently positive for aPL and that these aPL are potentially pathogenic.
To cite this abstract in AMA style:
Zuo Y, Estes S, Ghandi A, Yalavarthi S, Ali R, Hui S, Sule G, Gockman K, Madison J, Zuo M, Woodard W, Lezak S, Lugogo N, Kanthi Y, Knight J. Prothrombotic Antiphospholipid Antibodies in COVID-19 [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/prothrombotic-antiphospholipid-antibodies-in-covid-19/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prothrombotic-antiphospholipid-antibodies-in-covid-19/