Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: The management of fetuses exposed to maternal anti-SSA/Ro autoantibodies is challenging as the clinician weighs the rarity of fetal atrioventricular block (AVB) against the burden of serial surveillance. Antibody titer should be an important contributor to risk assessment and pathogenesis of disease since placental transport of maternal IgG via FcRn expressed on syncytiotrophoblast cells is less efficient during the second trimester with fetal IgG concentrations only about 10% of the maternal levels at weeks 17 – 22, the vulnerable period for AVB detection. Accordingly, we leveraged prospective data from the large multi-racial national study of pregnant women, Surveillance ToPrevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and utility of frequent ambulatory monitoring on outcomes in women with no previously affected children and those at risk for recurrence.
Methods: Women with positive anti-Ro autoantibodies by commercial CLIA testing from 21 sites across the U.S. were risk stratified into high and low anti-Ro60 and anti-Ro52 titers based on previous ELISA cutoff data demonstrating that women with titers < 1000 arbitrary units per mL were not at risk for fetal AVB. Those with low titers had echocardiograms only and EKGs at birth. Women exceeding this threshold for either anti-Ro60 or 52 performed fetal heart rate monitoring (FHRM) thrice daily in addition to weekly or biweekly fetal echocardiography from 18 – 26 weeks. Abnormal FHRM prompted urgent echocardiography to identify AVB.
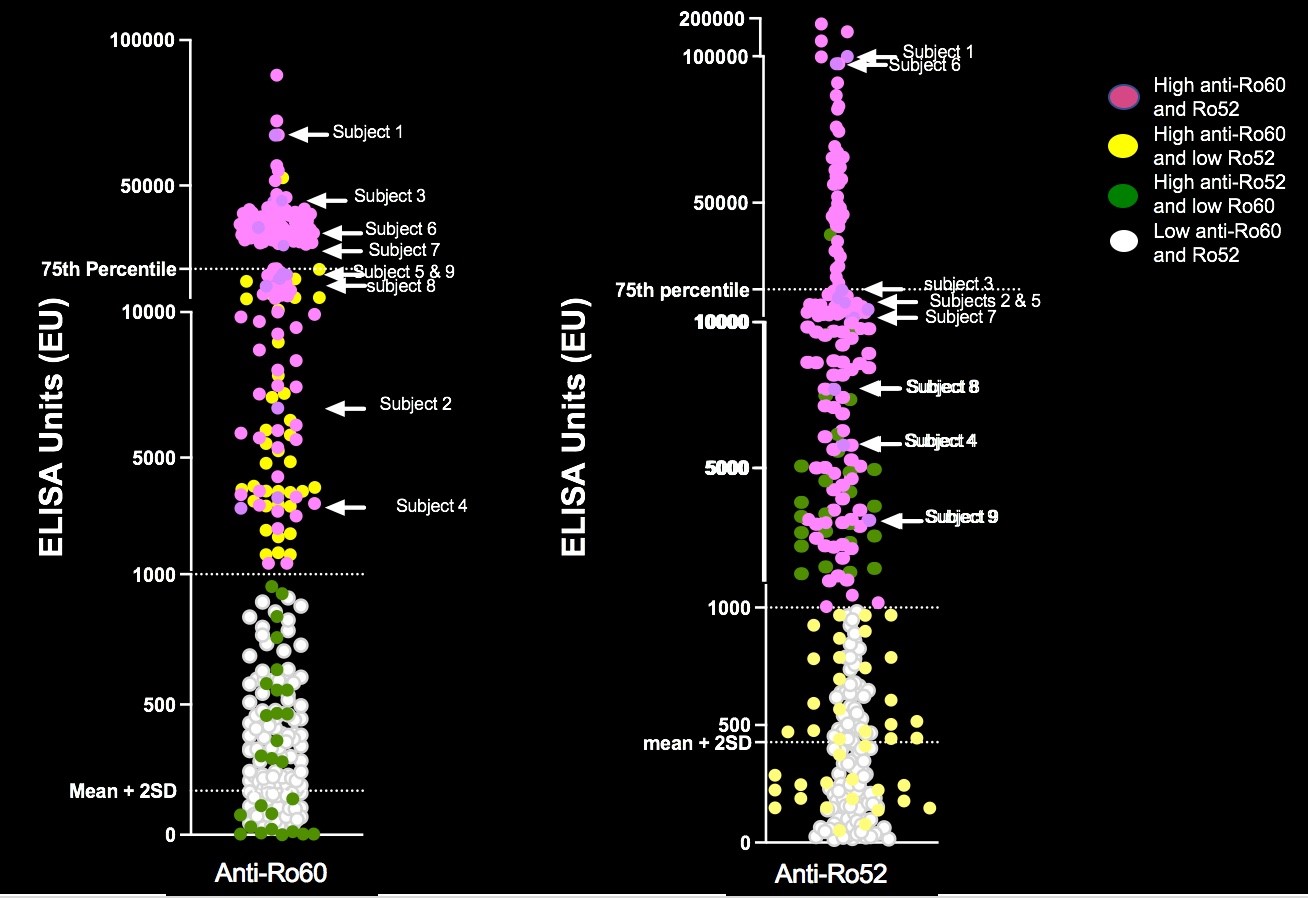
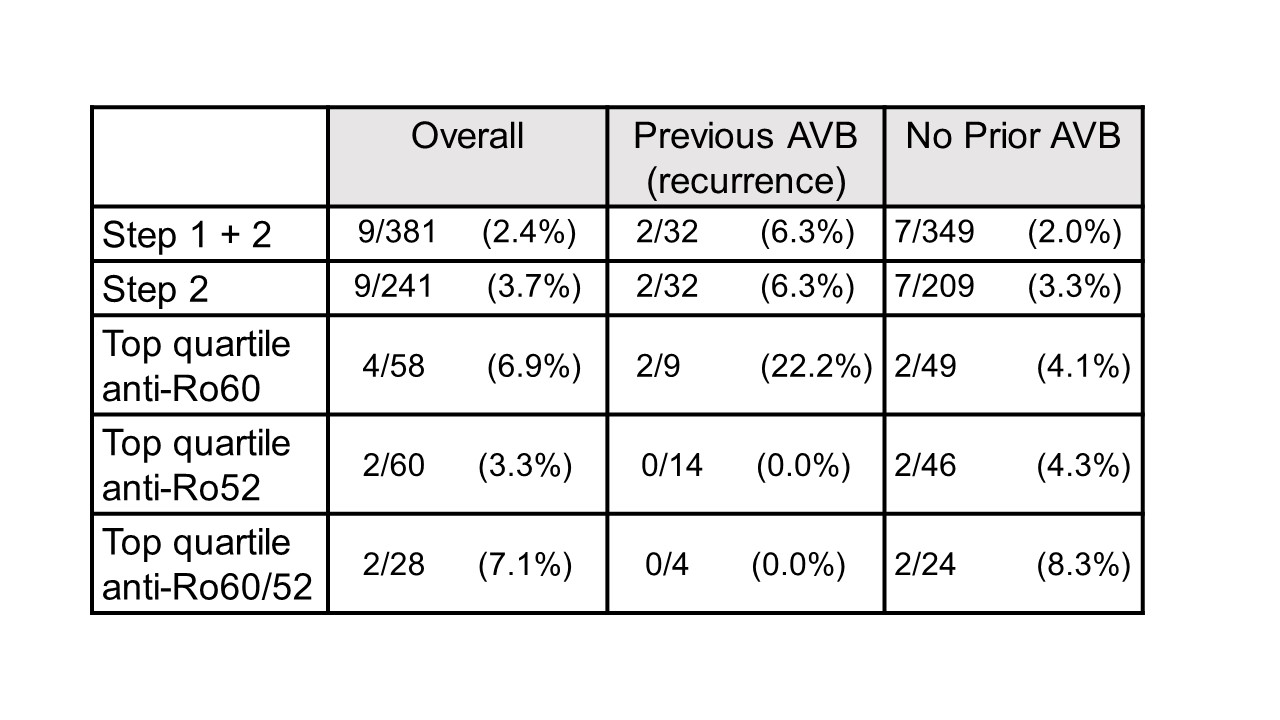
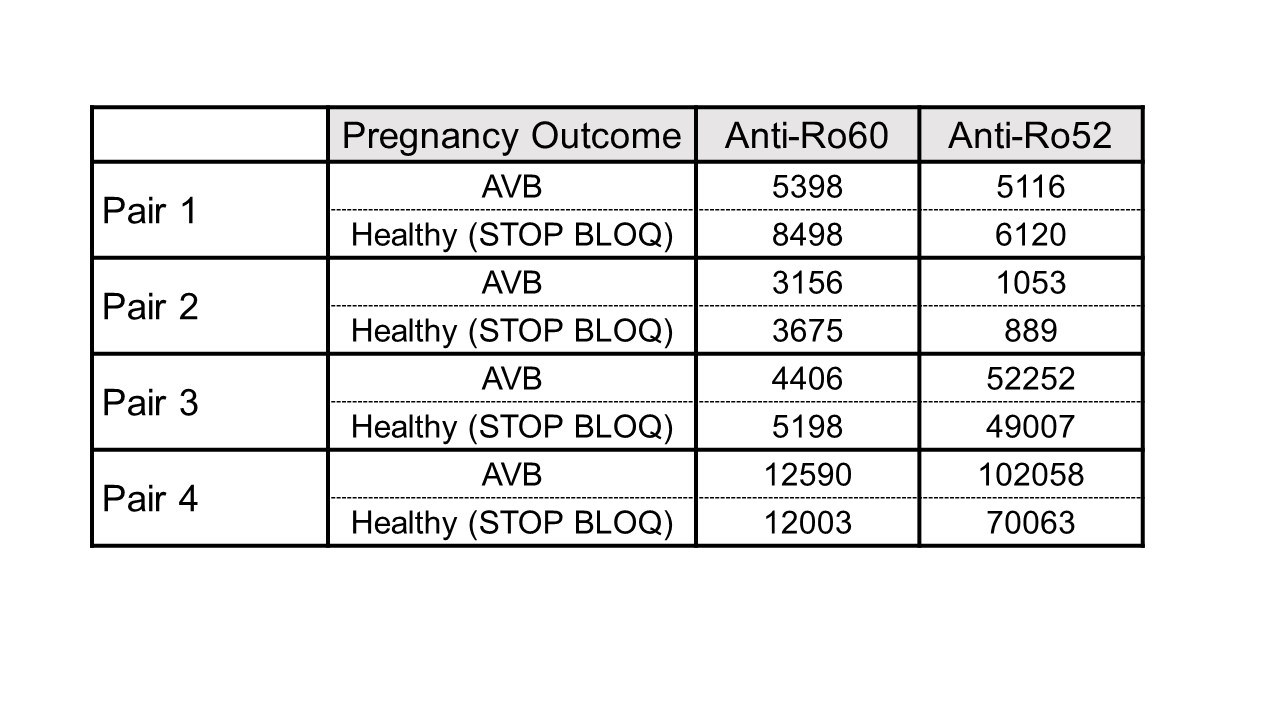
Results: To date, 405 women have been enrolled (Fig 1, titers). Of these, 150 (37%) had low titers of both anti-Ro60 and 52.None of 140 pregnancies past 26 weeks resulted in AVB. Of the 255 women with titers above the threshold for either antibody, 241 completed surveillance. FHRM (performed 44,187 times) was considered abnormal in 37 audios, 9 of which were confirmed AVB by urgent echo (7 were 2nd degree AVB). Surveillance echocardiogram (performed 1871 times) detected no AVB when the FHRM was normal. Of the 9 AVB, 2/32 (6.25%) were recurrences and 7/209 (3.3%) were first time AVB (Table 1). Independent of previous AVB status, comparing titers for the non AVB vs AVB in the high titer group, anti-Ro60 was 16155 + 19821 SD and 25133 + 21494 SD (P= 0.11) and anti-Ro52 was 21194 + 36165 SD and 32310 + 38871 SD (P = 0.04), respectively. AVB risk increased with titers (Table 1). For women within the top quartile for both anti-Ro60 and 52, the rate of AVB was 7.1% overall, and 8.3% for those never having AVB. Albeit limited numbers, having anti-Ro60 antibodies in the top quartile and a previous child with AVB conferred the highest risk of AVB, at 22%. High titer anti-Ro antibodies are necessary, but not sufficient for fetal AVB since 4 women with a prior pregnancy complicated by fetal AVB but without AVB in the current pregnancy had titers equivalent to those detected during their affected pregnancies (Table 2).
Conclusion: FHRM identifies early AVB, supporting this approach in the management of anti-Ro exposed pregnancies. To date, low titer anti-Ro confers no risk of AVB. While the titer of anti-Ro60 and 52 increases risk, as does previous AVB, additional factors beyond antibodies are likely.
To cite this abstract in AMA style:
Cuneo B, Masson M, Phoon C, Roman A, Izmirly P, Saxena A, Belmont M, Penfield C, Lee Y, Nusbaum J, Acherman R, Sinkovskaya E, Abuhamad A, Makhoul M, Satou G, Hogan W, Pinto N, Moon-Grady A, Howley L, Donofrio M, Krishnan A, Ahmadzia H, Levasseur S, Paul E, Owens S, Cumberback K, Matta J, Joffe G, Lindblade C, Rubenstein A, Haxel C, Kohari K, Copel J, Strainic J, Doan T, Bermudez-Wagner K, Sheth S, Killen S, Tacy T, Kaplinski M, Hornberger L, Fraser N, Clancy R, Buyon J. Prospective Evaluation of Anti-SSA/Ro Pregnancies Supports the Utility of High Titer Antibodies and Fetal Home Monitoring for the Detection of Fetal Atrioventricular Block [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/prospective-evaluation-of-anti-ssa-ro-pregnancies-supports-the-utility-of-high-titer-antibodies-and-fetal-home-monitoring-for-the-detection-of-fetal-atrioventricular-block/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prospective-evaluation-of-anti-ssa-ro-pregnancies-supports-the-utility-of-high-titer-antibodies-and-fetal-home-monitoring-for-the-detection-of-fetal-atrioventricular-block/