Session Information
Date: Sunday, November 8, 2020
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Connective tissue diseases (CTDs) are commonly identified causes for interstitial lung disease (ILD). Compared with idiopathic interstitial pneumonias, patients with CTD-ILD and interstitial pneumonia with autoimmune features (IPAF) are more likely to respond to immunosuppression with a better prognosis. We aim to investigate use of mycophenolate (MMF) and rituximab (RTX) in a mixed cohort of patients with CTD-ILD, prospectively followed up for one year in a single academic center.
Methods: Patients with CTD and ILD were identified based on electronic medical records from Mayo Clinic Jacksonville and Rochester campuses from 2009 – 2019. Chart review was conducted in 413 patients, with 138 confirmed cases of CTD-ILD or IPAF based on multidisciplinary evaluation. 91 were further excluded due to post transplant status, mild disease only on observation, treatment with agents other than MMF and RTX, or incomplete data. Eventually, 47 patients with CTD-ILD or IPAF, treated with MMF and / or RTX, were included. Demographics, pertinent clinical symptoms, pulmonary function test (PFT) and treatment were collected at baseline, 6-month and 12-month follow up.
MMF and RTX doses and treatment track were analyzed individually with improvement in PFT. Multivariable linear regression models were used to assess baseline and 6-month treatment doses of MMF and RTX, with changes in outcomes during 12-month follow-up. All models were adjusted for age, sex, and prednisone dose at corresponding follow-ups.
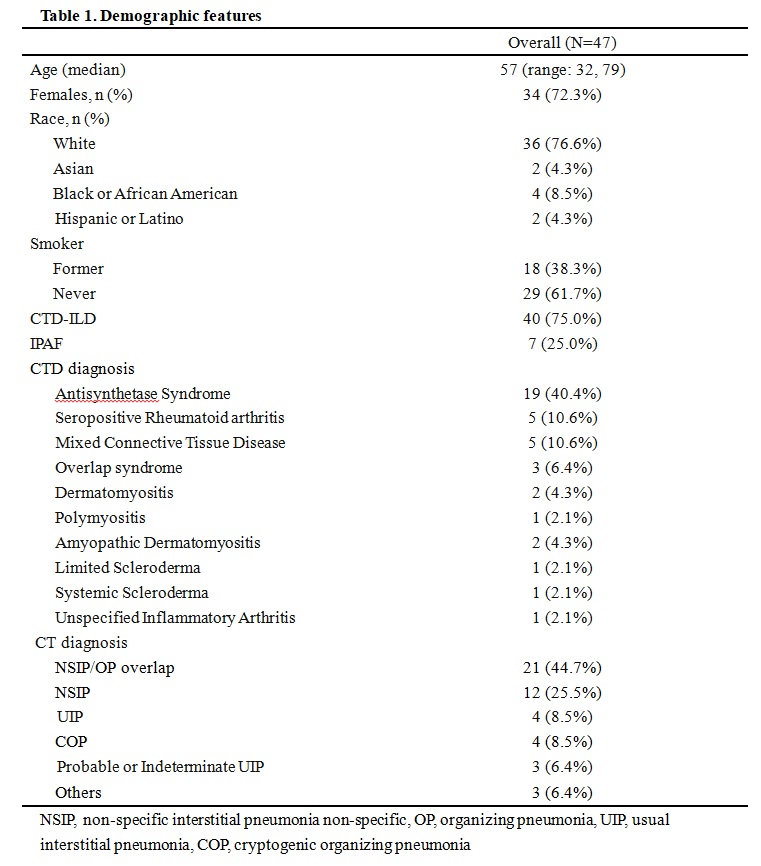
Results: Baseline characteristics of the 47 patients are summarized in Table 1. Forty (85%) patients were diagnosed with CTD-ILD, with 19 (40%) as Antisynthetase Syndrome, and 7 (15%) had IPAF. Six (13%) patients were on RTX only, 28 (60%) on MMF only, and the remaining 13 (28%) were on combined therapy with MMF and RTX or switched from MMF to RTX due to lack of response or intolerance to MMF.
PFT outcomes in association with medication dose are summarized in Table 2. Throughout the 12-month study period, forced vital capacity (FVC) and FVC% remained stable with each treatment dose or track. Patients on higher doses of MMF were associated with an average increase of 1.34 (95% CI: 0.38, 2.29) units in diffusing capacity for carbon monoxide (DLCO) for every 1-gram increase in MMF, from baseline to 6-month (P=0.01), which stabilized from 6 to 12-month follow-up. Patients on RTX monotherapy had significantly worsening in DLCO by 7.21 (95% CI: 4.08, 10.33) units (P< 0.001) from baseline to 12-month.
Ten patients received MMF and RTX combined therapy during study period, with clinical features summarized in Table 3. Although pulmonary function was severely compromised at baseline, these patients acquired sustained improvement from baseline to 12-month. In all cases, prednisone was decreased to a maintenance average dose of 8 mg/day at 12-month follow up.
Conclusion: Through the study period, patients with CTD-ILD or IPAF, treated with MMF, presented with stability of FVC and significant improvement in DLCO; while RTX monotherapy was associated with worsening DLCO. Combination of both medications was related with sustained improvement in PFT and gradual decrease in concurrent steroids doses.
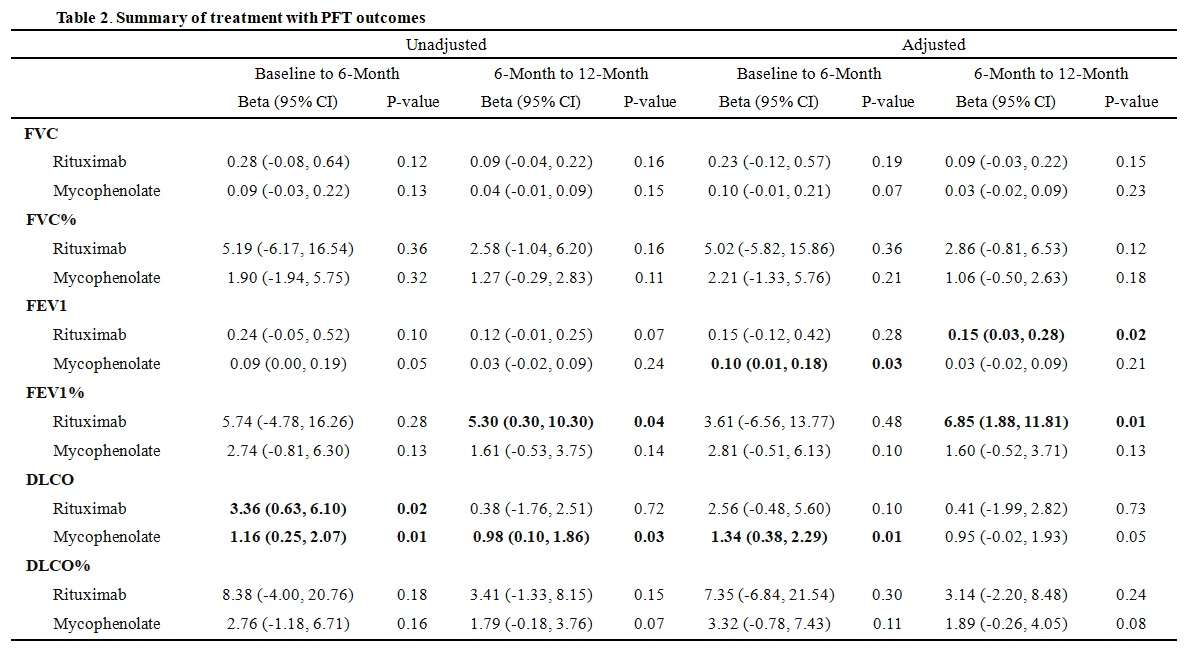 Table 2. Summary of treatment with PFT outcomes
Table 2. Summary of treatment with PFT outcomes
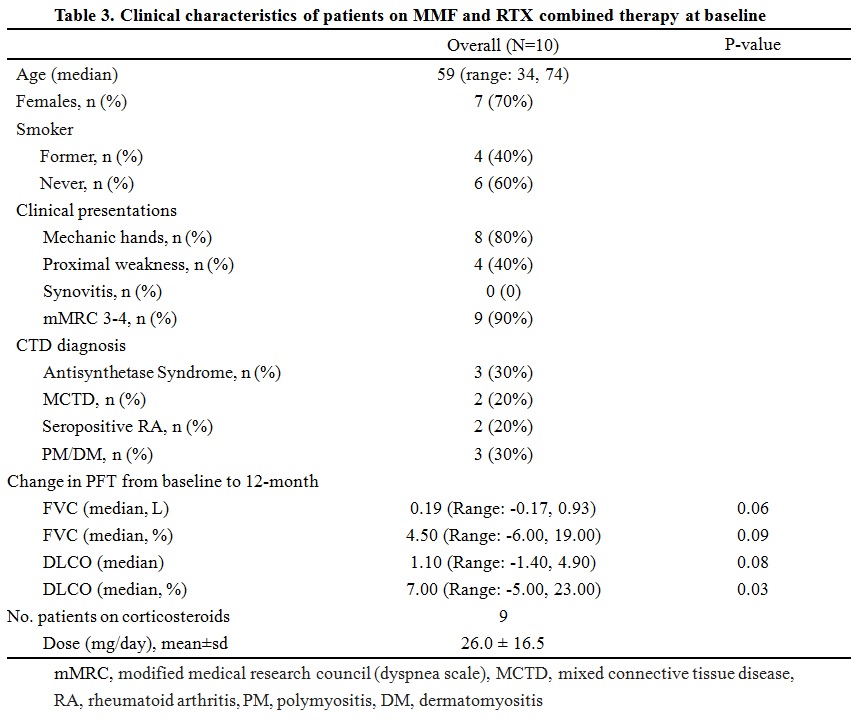 Table 3. Clinical characteristics of patients on MMF and RTX combined therapy at baseline
Table 3. Clinical characteristics of patients on MMF and RTX combined therapy at baseline
To cite this abstract in AMA style:
Li Y, Baig H, Rojas C, Stowell J, Lesser E, Borkar S, Abril A, Mira-Avendano I. Prospective Analysis of a Cohort of Patients with Interstitial Lung Disease Associated with Connective Tissue Disease and Their Response to Immunosuppression with Mycophenolate Mofetil and Rituximab [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/prospective-analysis-of-a-cohort-of-patients-with-interstitial-lung-disease-associated-with-connective-tissue-disease-and-their-response-to-immunosuppression-with-mycophenolate-mofetil-and-rituximab/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prospective-analysis-of-a-cohort-of-patients-with-interstitial-lung-disease-associated-with-connective-tissue-disease-and-their-response-to-immunosuppression-with-mycophenolate-mofetil-and-rituximab/