Session Information
Date: Tuesday, October 23, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: On regulatory approval in India, challenging socioeconomics and infection prone scenario compelled us to seek prolonged effectiveness of short term anti-TNF therapeutic regimen (Chopra et al. APLAR Congress 2005). However, there was very little professional and industrial support to research this approach. With the advent of ‘Biosimilars’ ,we decided to evaluate the effectiveness of a shorter therapeutic regimen of a Biosimilar Adalimumab in AS
Methods: 62 consenting severely symptomatic patients (86% B 27+) with failed NSAID response were screened;12 were suspected latent TB and offered INH prophylaxis. 50 patients (42 males, mean age 31.2 years, mean duration 98.8 months) were enrolled into an observational design study in a community practise setting: mean of AS-DAS 4.6, ESR 88 mm, CRP 64 mg/dl (nephelometry, cut off 5 mg/dl). 40 mg Biosimilar Adalimumab (Bsmr-ADL) (Exemptia™) was administered sub cutaneous every fortnight for 12 weeks as per protocol and standard (ACR) clinical and laboratory monitoring performed. cytokines assay Standard intention-treat analysis was performed (Student T and matched sign rank); significant p <0.05.
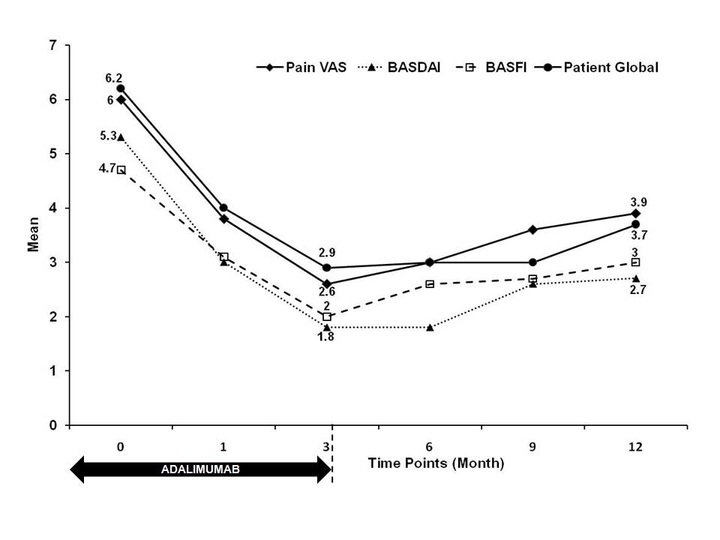
Results: Improvement was rapid (week 4- mean ESR 29.1 mm), significant and sustained (Figure). The Tables shows the proportion of patients showing index improvement and ASDAS change. 10 patients failed ASAS 20 at week 12 and despite additional Bsmr-ADL (2 injections, 2 week apart) did not show ASAS based improvement at later time points (data not shown). 12 patients withdrew (1 drug fear,4 logistics, 5 poor response, 2 unknown). None had active TB/severe AE. Cytokine assay (IL-6, TNF α and IL-17) at baseline and a-priori endpoints will be presented. We continue to monitor patients and have completed 18 months post ADA. We lacked active control and did not study structure modification.
Conclusion: This investigator initiated study demonstrated prolonged benefit of a 12 week regimen with Biosimilar ADA in several patients of severe AS. This is a promising way forward in our setting. But validation studies are required..
Acknowledgment: Zydus Cadilla India provided a generous research grant and substantial free of cost Biosimilar ADL injections.
Table shows the proportion of patients with severe AS showing response at study end points
|
Index |
12-14 weeks |
22-26 weeks |
46-52 weeks |
|
ASAS 20 (%) |
80 |
72 |
52 |
|
ASAS 40(%) |
68 |
60 |
38 |
|
ASDAS (mean) |
2.4 |
2.9 |
3.5 |
|
ASAS partial remission (%) |
34 |
22 |
12 |
Figure shows the mean Efficacy Measures in severe AS treated with Biosimilar Adalimumab
To cite this abstract in AMA style:
Chopra A, Khadke N, Saluja M, Kainifard T, Venugopalan A. Prolonged Effectiveness of a 12 Week Regimen of Biosimilar Adalimumab in Indian (Asian) Patients Suffering from Symptomatic Acute-Chronic Ankylosing Spondylitis (AS) [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/prolonged-effectiveness-of-a-12-week-regimen-of-biosimilar-adalimumab-in-indian-asian-patients-suffering-from-symptomatic-acute-chronic-ankylosing-spondylitis-as/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prolonged-effectiveness-of-a-12-week-regimen-of-biosimilar-adalimumab-in-indian-asian-patients-suffering-from-symptomatic-acute-chronic-ankylosing-spondylitis-as/