Session Information
Date: Sunday, November 13, 2022
Title: Systemic Sclerosis and Related Disorders – Clinical Poster II
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Short disease duration is a well known predictor for progressive systemic sclerosis-associated interstitial lung disease (SSc-ILD), but studies assessing ILD progression in later disease stages are lacking. To individually tailor management of ILD in SSc patients in clinical practice it is, however, of high importance to understand disease behaviour also in patients with late stage disease. The objective was to analyse ILD progression in SSc-ILD patients from the EUSTAR cohort segregated by subgroups of disease duration.
Methods: We segregated SSc-ILD patients into four categories of disease duration (≤3 years, >3-≤7 years, >7-≤15 years and >15 years after onset of Raynaud’s phenomenon). We assessed progressive ILD, defined as absolute forced vital capacity (FVC) decline >10% or FVC decline ≥10% and FVC decline 5–10% and diffusing capacity of the lungs for carbon monoxide (DLCO) decline ≥15% (composite decline) over the first and second 12+/-3 months period after first registration (baseline) into EUSTAR. Clinical characteristics, pulmonary involvement, treatment at first registration and ILD progression were evaluated by descriptive statistics.
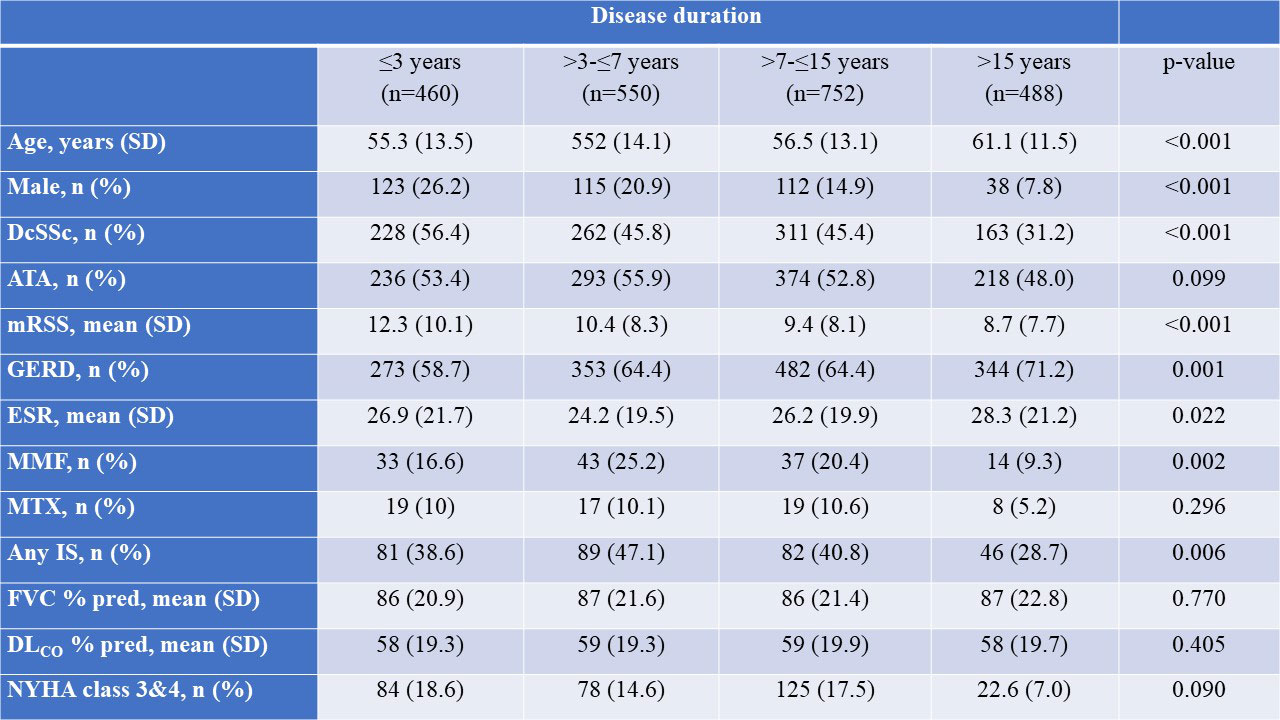
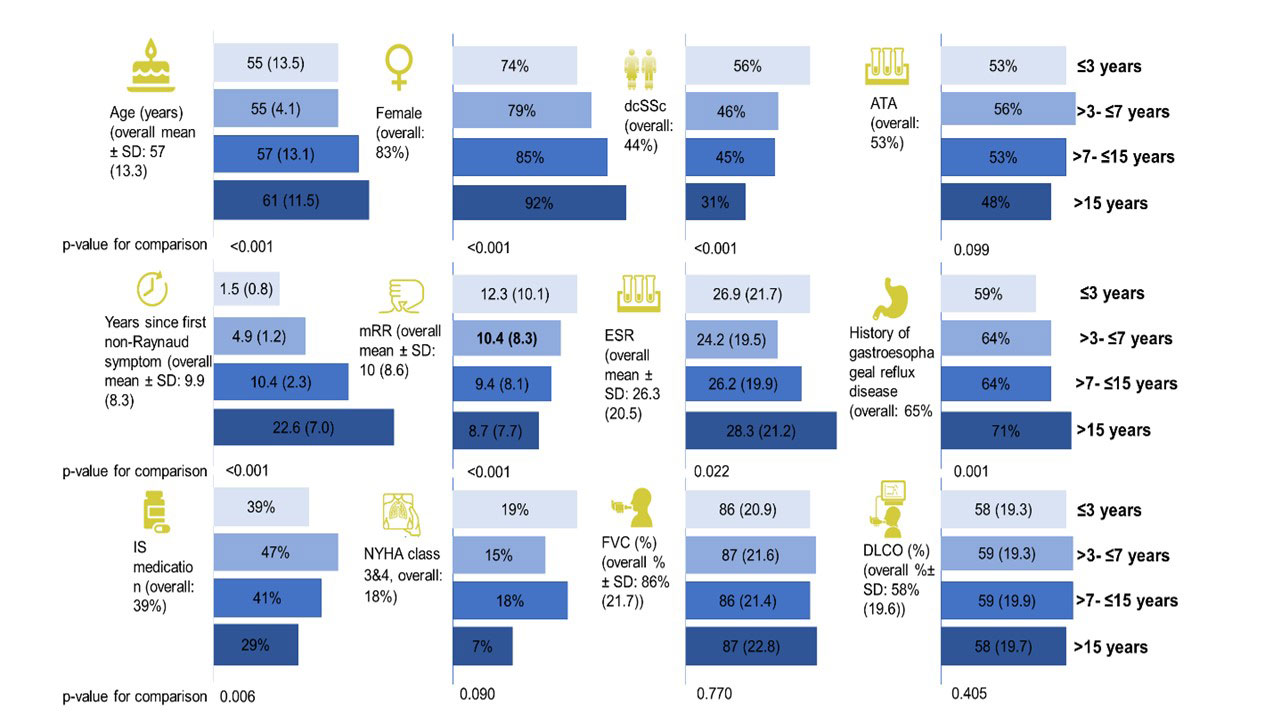
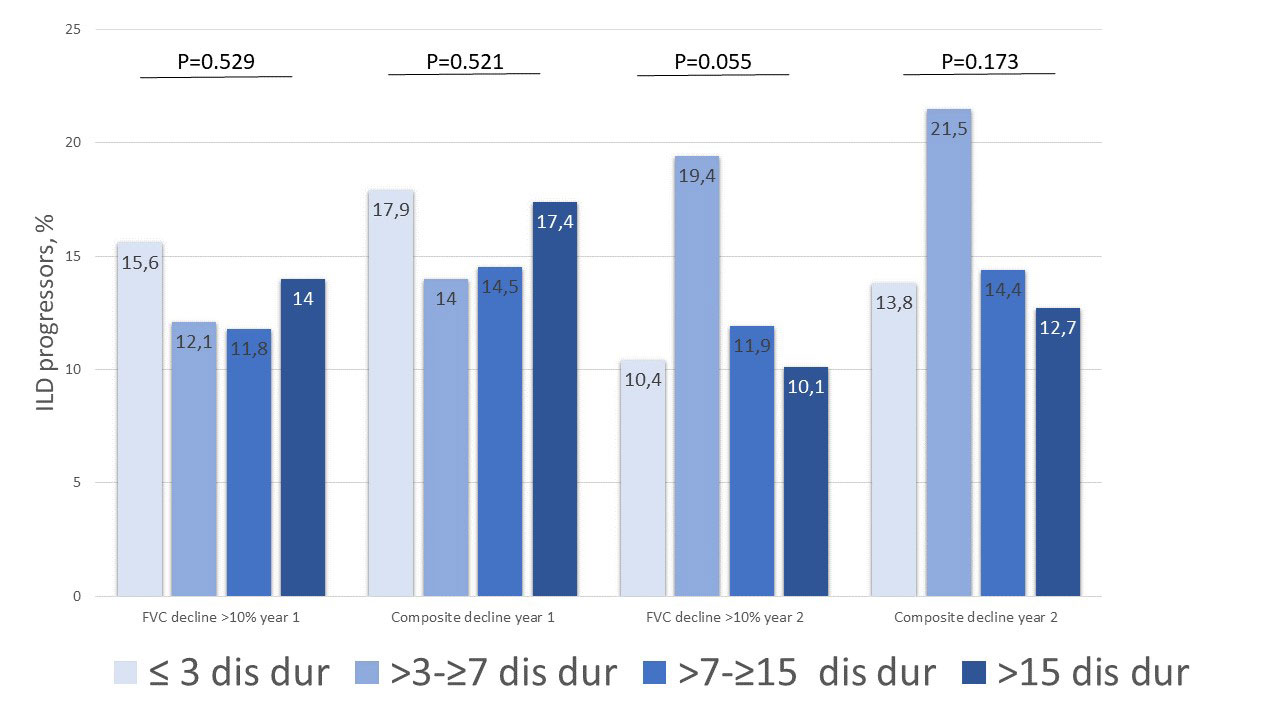
Results: In total, 2258 SSc-ILD patients were included, with 469 (20.8%) having a disease duration ≤3 years, 550 (24.4%) between >3-≤7 years, 752 (33.3%) between >7-≤15 years and 488 (21.6%) of >15 years (Table). Baseline characteristics and treatment patterns differed between the four subgroups, with more younger male patients with diffuse cutaneous SSc, anti-topoisomerase I antibody and higher Rodnan skin score having ≤3 years disease duration (Figure 1). Lung function with FVC and DLCO were similar between the four groups (Table). Notably, in the first and second 12+/-3 months periods after first registration in the EUSTAR database, there were no significant difference in FVC decline >10% or composite FVC and DLCO decline within the four subgroups. For example, patients with disease duration >7-≤15 years and >15 years frequently showed disease progression of FVC >10%: 41/347 (11.8%) and 32/228 (14%) compared to 38/244 (15.6%) and 33/273 (15.6%) for disease duration ≤3 years and >3-≤7 years (P=0.529), respectively (Figure 2).
Conclusion: It was long believed that ILD burned out in late disease stages. In our analysis of ILD progression by four disease duration categories, we showed that ILD frequently progressed also in late disease stages. This has important implications for clinical practise, as SSc patients need to be regularly monitored for ILD progression independent of disease duration.
To cite this abstract in AMA style:
Hoffmann-Vold A, Brunborg C, Airò P, Ananyeva L, Czirják L, Guiducci S, Hachulla E, LI M, Mihai C, Riemekasten G, Sfikakis P, Valentini G, Kowal-Bielecka O, Allanore Y, Distler O. Progressive Interstitial Lung Disease Is Frequent Also in Late Disease Stages in Systemic Sclerosis Patients from EUSTAR [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/progressive-interstitial-lung-disease-is-frequent-also-in-late-disease-stages-in-systemic-sclerosis-patients-from-eustar/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/progressive-interstitial-lung-disease-is-frequent-also-in-late-disease-stages-in-systemic-sclerosis-patients-from-eustar/