Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Background: There is an increasing knowledge about the utility of urinary biomarkers for the diagnosis of lupus nephritis (LN) in patients with systemic lupus erythematosus (SLE). However, information as prognostic factors is more limited. Our aim was to evaluate a wide panel of urinary biomarkers in patients with SLE in order to assess their capability for prediction of progression to end stage renal disease (ESRD) in LN patients.
Methods: We conducted a prospective single center study including patients with SLE. Only those with LN criteria according ACR were included. A wide panel of urinary biomarkers were measured at the moment of study inclusion, then, a prospective follow up was made according daily clinical practice. We measured urinary levels of 6 different biomarkers including: monocyte chemoattractant protein 1 (MCP-1), neutrophil gelatinase-associated lipocalin (NGAL), TWEAK, Ceruloplasmin (CP), Transferrin (TF) and vascular cell adhesion molecule-1 (VCAM-1) using a commercial ELISA kits (R&D system and Assaypro, USA). In addition, serum anti C1q antibodies were measured by ELISA (Inova, USA).
Continuous data were summarized as means ± SDs and compared by an independent T test or Mann–Whitney test. Categorical data were expressed as number of patients and percentages and compared by Fisher’s exact test. Receiver operating characteristic (ROC) curve analysis was used to explore discrimination between those with ESRD and determine the optimal cutoff point for different biomarkers. Survival curves were drawn using the Kaplan–Meier method. Statistical analyses were performed using IBM SPSS Statistics version 23.0.
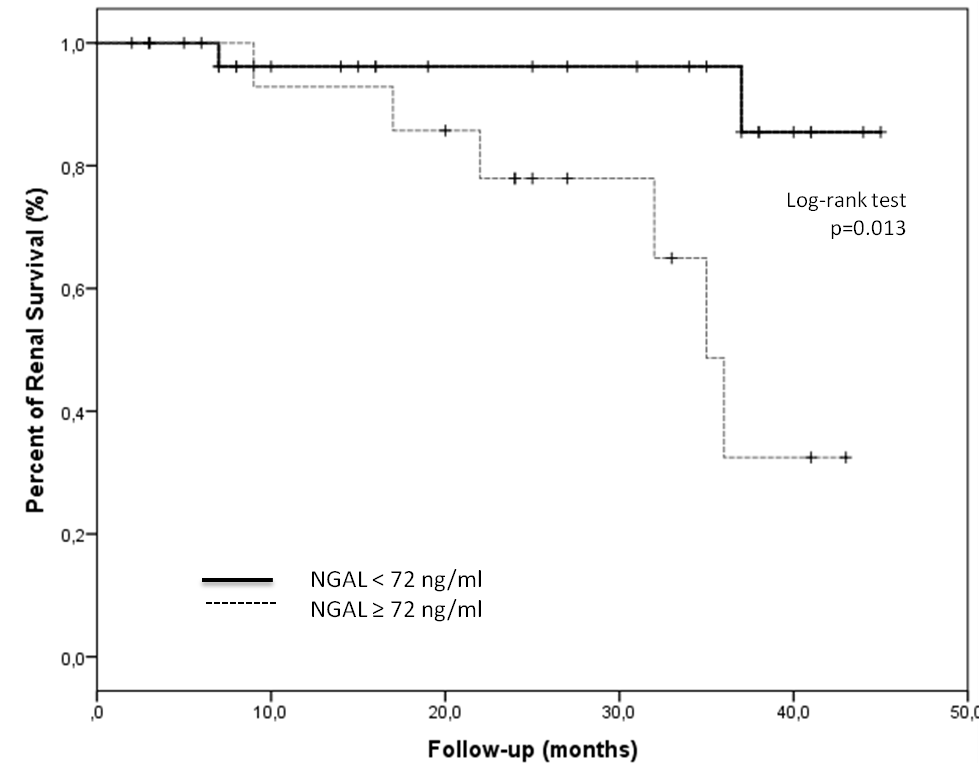
Results: From 120 patients with SLE, 76 (63%) had LN. The median age of patients at time of inclusion was 29.3 ± 9.4 years and the average follow-up period was 23.0 ± 13.1 months. Main clinical and serological characteristics are summarised in Table. During the follow-up 4 patients had LN progression (From class II to Class IV or V). Two patients had chronic renal disease at baseline. Eight out of 76 (10.5 %) progressed to ESRD, and 2 patients deceased. Urinary levels of NGAL (434.5 ± 193.0 vs 73.7 ± 21.5 ng/ml) and TF (8528.5 ± 234.2 vs 6780 ± 539 ng/ml) were significantly higher in those patients who progressed to ESRD. No differences were found with other urinary biomarkers or serum Anti C1q levels and risk of ESRD. The combination of these NGAL and TF compared to any of the urine markers individually improved the prediction of future ESRD. AUC values were 0.71 and 0.63, respectively. Comparisons of renal survival grouped by the level of urinary NGAL at baseline were predictors for ESRD in LN patients (Figure).
Conclusion: In this cohort of patients with LN, around 10% of patients progressed to ESRD. High levels of Urinary NGAL levels at baseline were related with a higher risk of progression to ESRD.
To cite this abstract in AMA style:
Gómez-Puerta J, Urrego T, Ortiz Reyes B, Vanegas-García A, Muñoz-Vahos C, González L, Vasquez G. Prognostic Value of Urinary Biomarkers for the Developing of End Stage Renal Disease in Patients with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/prognostic-value-of-urinary-biomarkers-for-the-developing-of-end-stage-renal-disease-in-patients-with-systemic-lupus-erythematosus/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prognostic-value-of-urinary-biomarkers-for-the-developing-of-end-stage-renal-disease-in-patients-with-systemic-lupus-erythematosus/