Session Information
Session Type: Abstract Submissions (ACR)
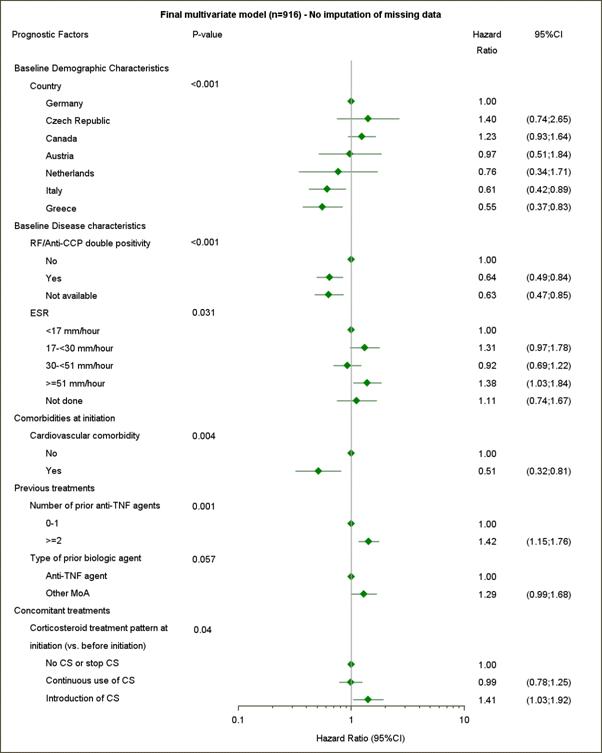
Results: 1009/1131 (89.2%) evaluable pts had failed ≥1 prior biologic agent. The crude retention rate (95% CI) at 24 months (Kaplan–Meier method) for pts exposed to ≥1 prior biologic agent was 53.4% (50.1, 56.6%).1 995 of 1009 pts were included in the analysis of prognostic factors. Final multivariate model results (n=916) are shown in the Figure. Pts had significantly higher likelihood of ABA retention if they were both RF and ACPA positive or had cardiovascular comorbidity at initiation. Prior anti-TNF agents, high baseline ESR and corticosteroid (CS) use were also prognostic factors for discontinuation. Despite showing borderline significance in the first model (Figure), use of a non-anti-TNF biologic agent before ABA (n=143, 15.6%) was an additional prognostic factor of lower retention (1.29 [1.00, 1.66]; p=0.049) in the model with imputation of missing data (not shown). Disease duration, ABA monotherapy and BMI were not identified as prognostic factors.
Conclusion: ACTION is one of the first studies to identify and report prognostic factors of long-term abatacept retention in a real-world setting. Double ACPA and RF positivity and cardiovascular comorbidity at initiation were prognostic of higher retention. Consistent with other reports,2,3 higher number of prior anti-TNFs, country and more severe disease (suggested by higher baseline ESR and introduction of CS) were identified as prognostic factors of lower retention. These results will support individualized biologic treatment strategies in pts with moderate-to-severe RA.
1. Nüßlein H, et al. Ann Rheum Dis 2014;73:(S2):FRI0318.
2. Finckh A, et al. Arthritis Rheum 2013;65(S10):S217.
3. Neto D, et al. Arthritis Rheum 2013;65(S10):S1248.
Disclosure:
H. Nüßlein,
Bristol-Myers Squibb, Abbott, Chugai, UCB, Essex, Wyeth, Pfizer, MSD, Novartis and Roche,
5;
R. Alten,
Bristol-Myers Squibb,
2,
Bristol-Myers Squibb,
5;
M. Galeazzi,
None;
H. Lorenz,
Bristol-Myers Squibb,
5;
M. Nurmohamed,
BMS, Janssen,
5,
Roche, Abbvie, Pfizer, UCB,
8,
Roche, Abbvie, Pfizer, MSD, UCB, BMS,
2;
W. Bensen,
Abbott, Amgen, BMS, Janssen, Merck, Lilly, Novartis, Pfizer, Proctor and Gamble, Roche, Sanofi -Aventis, Schering, Takeda, UCB, Warner Chilcott, Wyeth,
2,
Abbott, Amgen, BMS, Janssen, Merck, Lilly, Novartis, Pfizer, Proctor and Gamble, Roche, Sanofi -Aventis, Schering, Takeda, UCB, Warner Chilcott, Wyeth,
5,
Abbott, Amgen, BMS, Janssen, Merck, Lilly, Novartis, Pfizer, Proctor and Gamble, Roche, Sanofi -Aventis, Schering, Takeda, UCB, Warner Chilcott, Wyeth,
8;
G. Burmester,
AbbVie, Pfizer, Roche, UCB,
2,
AbbVie, BMS, MSD, Medimmune, Novartis, Pfizer, Roche, Sandoz, UCB,
5,
AbbVie, BMS, MSD, Pfizer, Roche, Sandoz, UCB,
8;
H. H. Peter,
None;
P. Peichl,
None;
K. Pavelka,
MSD, AbbVie, Pfizer, UCB, Roche, Amgen, Menarini, BMS,
5;
M. Chartier,
None;
C. Poncet,
Bristol-Myers Squibb,
9;
C. Rauch,
Bristol-Myers Squibb,
3;
M. Le Bars,
Bristol-Myers Squibb,
3,
Bristol-Myers Squibb,
1.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prognostic-factors-for-iv-abatacept-retention-in-patients-who-have-received-at-least-one-prior-biologic-agent-2-year-results-from-a-prospective-international-real-world-study/