Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Progesterone plays a protective role in preventing inflammation and preterm delivery during pregnancy. However, the mechanism involved is unknown. Microbial product translocation from a permeable mucosa is demonstrated as a driver of inflammation.
Methods: To study the mechanism of the protective role of progesterone during pregnancy, we investigated the effect of physiologic concentrations of progesterone on tight junction protein occludin expression and human gut permeability in vitro and systemic microbial translocation in pregnant women in vivo. Plasma bacterial lipopolysaccharide (LPS), a representative marker of in vivo systemic microbial translocation was measured. Increased microbial translocation from a “leaky” gut has been shown to play a role in autoimmune diseases and pre-term delivery.
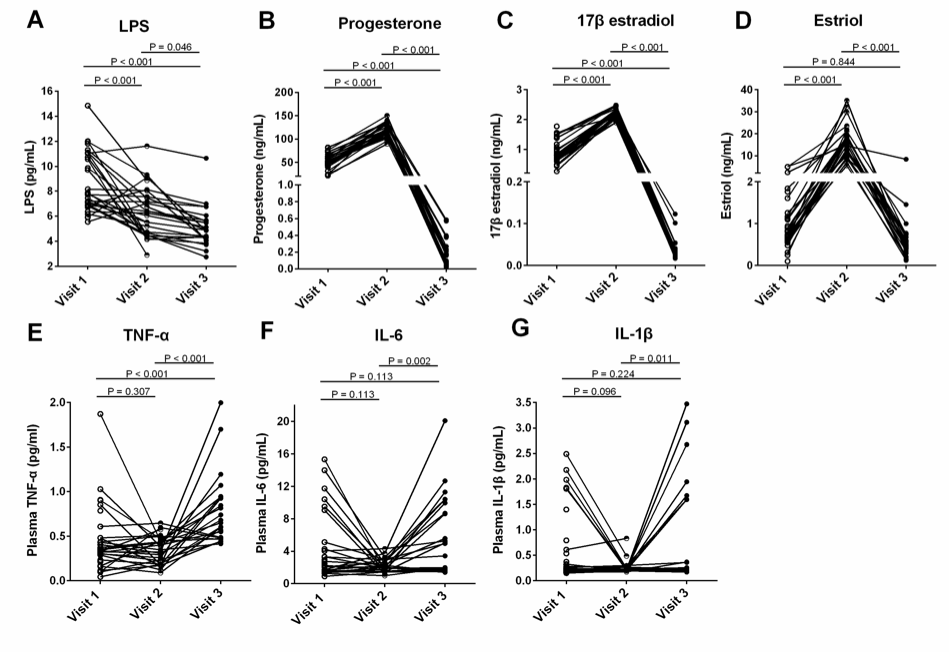
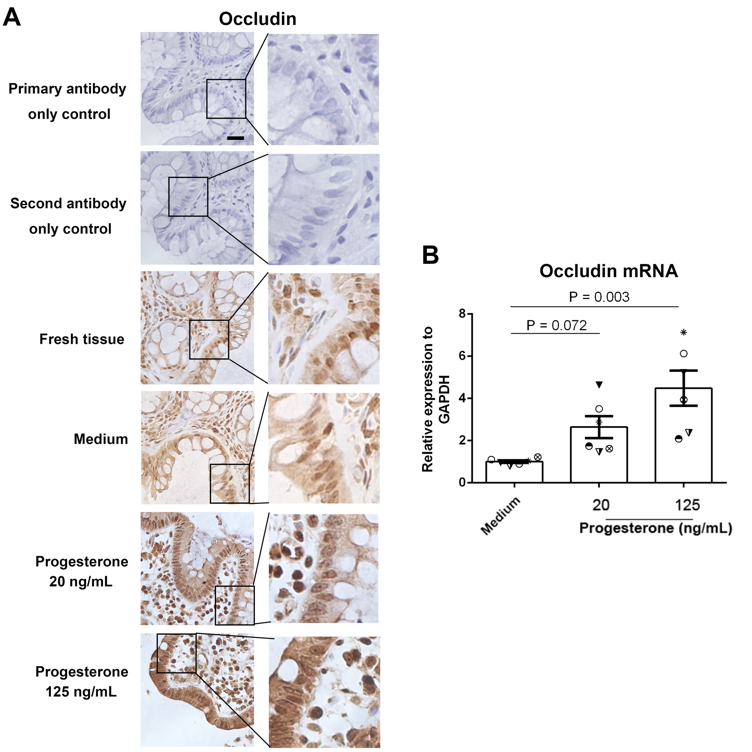
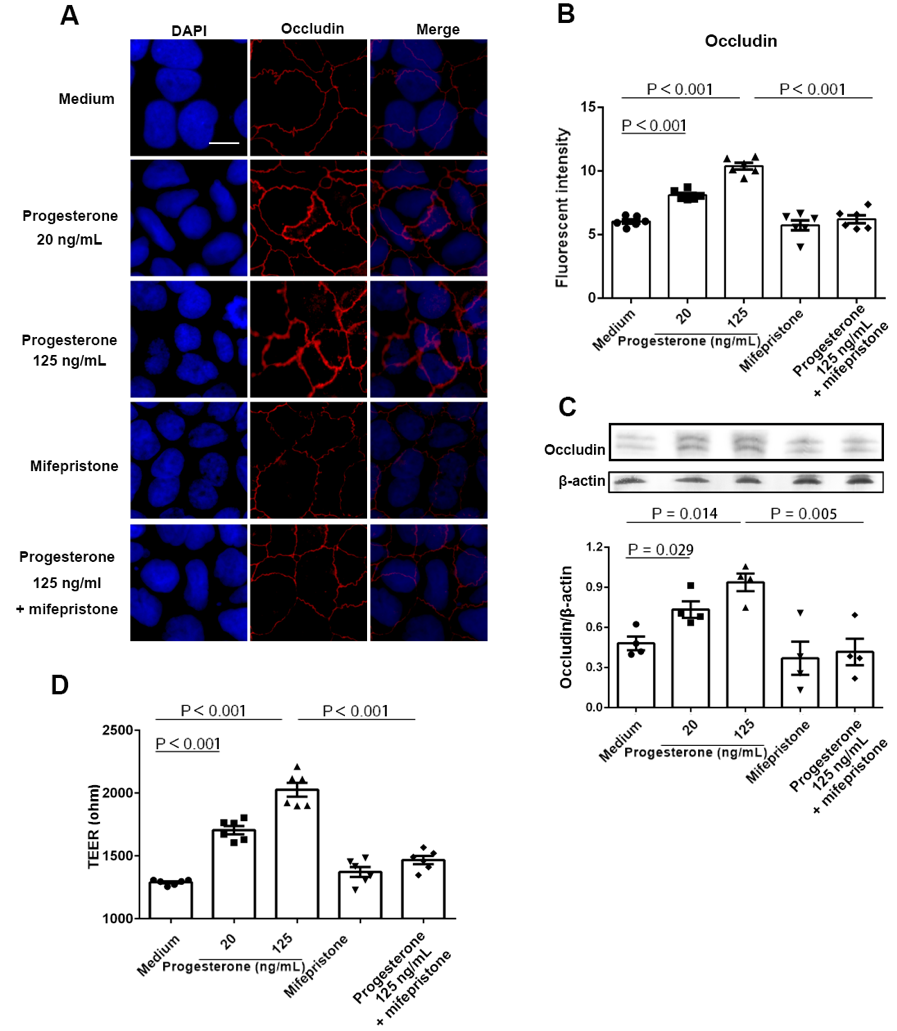
Results: We found that plasma LPS levels were significantly decreased from 24 to 28 weeks of gestation compared to 8 to 12 weeks of gestation (P < 0.05, ANOVA). The median plasma levels of LPS (pg/mL) were 7.76 (6.86 – 11.04) and 6.25 (4.53 – 7.55) and 4.88 (4.10 – 5.66) for 8-12 (visit 1), 24-28 weeks (visit 2) of gestation, and 6 to 8 weeks postpartum (visit 3), respectively. Plasma estriol and progesterone levels were increased at visit 2 compared to visit 1 but decreased at visit 3 (P < 0.05, non-parametric Mann-Whitney test). Moreover, plasma LPS levels were negatively correlated with plasma progesterone levels but positively correlated with plasma tumor necrosis factor-alpha (TNF-α) levels at visit 1 (Spearman correlation test) but not at visit 2. Progesterone treatment increased intestinal trans-epithelial electrical resistance (TEER) in primary human colon tissues and Caco-2 cells in vitro through upregulating tight junction protein occludin expression using IHC, qPCR, and TEER assays (P < 0.05, non-parametric Mann-Whitney test). Furthermore, progesterone exhibited an inhibitory effect on nuclear factor kappa B (NF-κB) activation following LPS stimulation in Caco-2 cells by IHC, western blot and qPCR (P < 0.05, non-parametric Mann-Whitney test).
Conclusion: These results reveal a novel mechanism that progesterone may play an important role in decreasing mucosal permeability, systemic microbial translocation, and inflammation during pregnancy.
To cite this abstract in AMA style:
Zhou Z, Gilkeson G, Kamen D, Oates J, Jiang W. Progesterone Decreases Gut Permeability Through Upregulating Occludin Expression in Primary Human Gut Tissues and Caco-2 Cells [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/progesterone-decreases-gut-permeability-through-upregulating-occludin-expression-in-primary-human-gut-tissues-and-caco-2-cells/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/progesterone-decreases-gut-permeability-through-upregulating-occludin-expression-in-primary-human-gut-tissues-and-caco-2-cells/