Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Procoagulant activity is an important property of EVs. Connective tissue disease (CTD) patients with livedo reticularis (LR) are known for their procoagulant conditions. The objective of this study is to investigate the role of EVs in LR.
Methods: We isolated EVs from plasma of 12 LR patients and 7 controls using sequential ultracentrifugation and size-exclusion chromatography. The EVs were analyzed by protein mass spectrometry. We measured tissue factor (TF) procoagulant activity and analyzed the cellular origin of EVs.
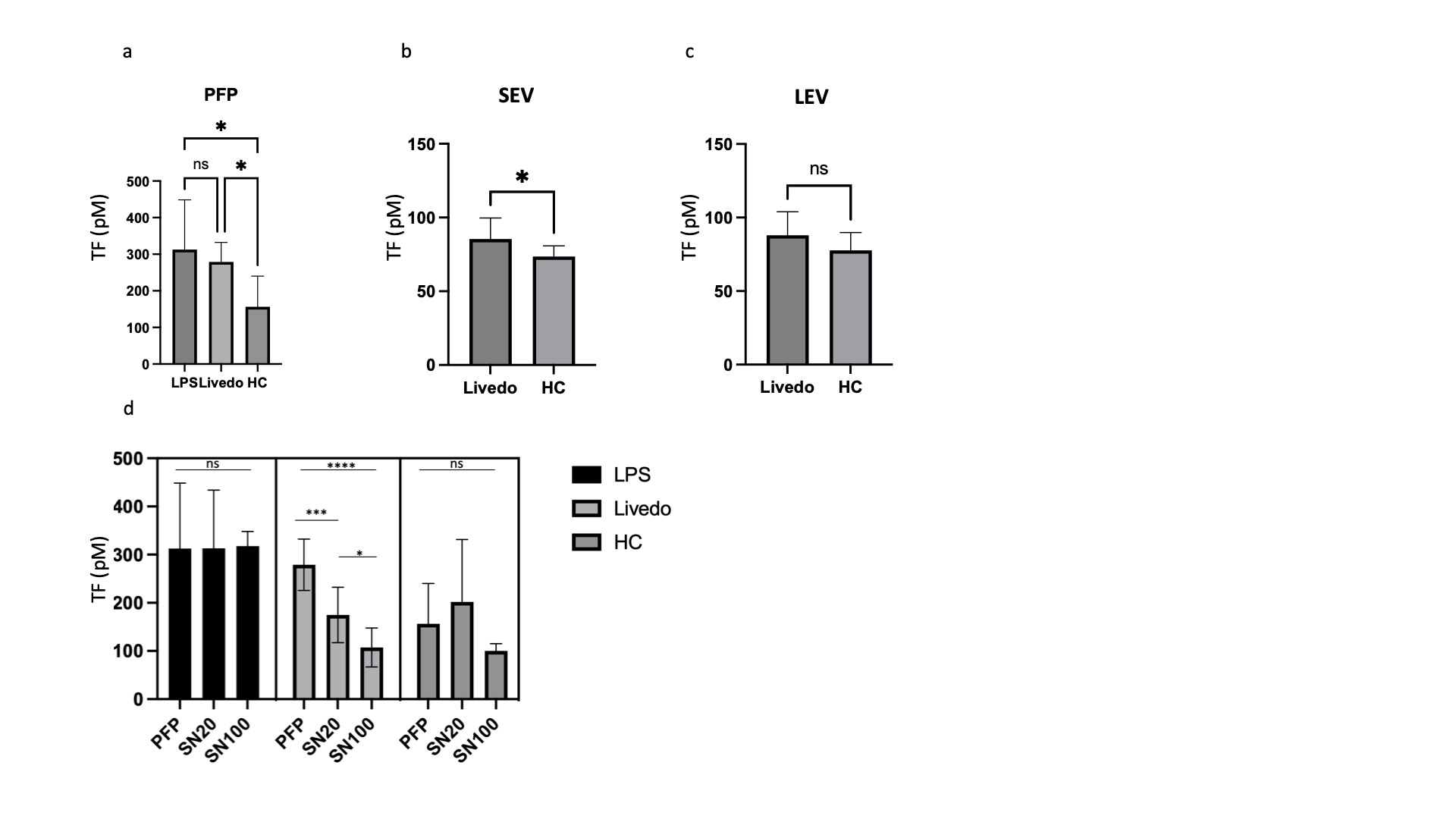
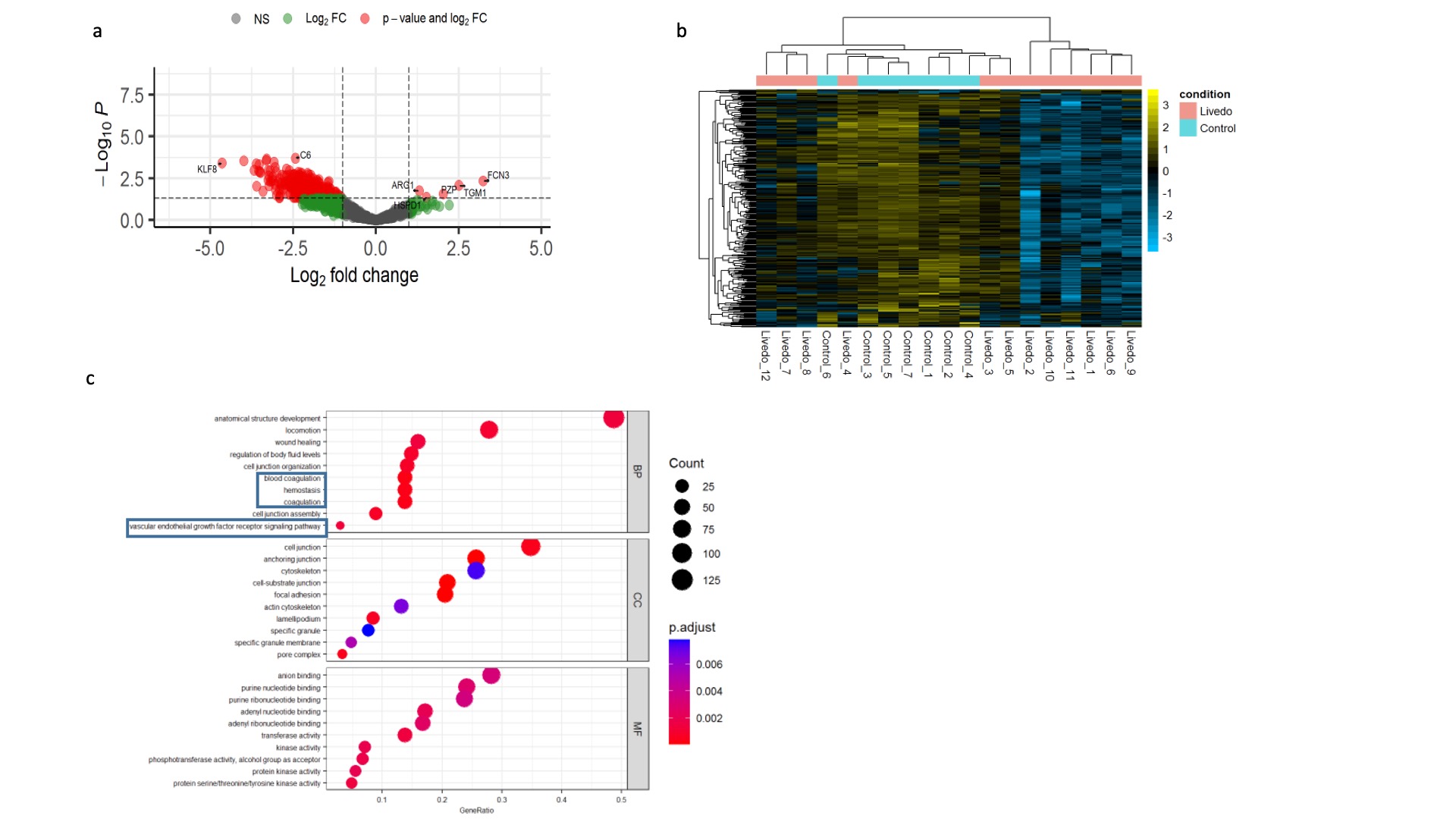
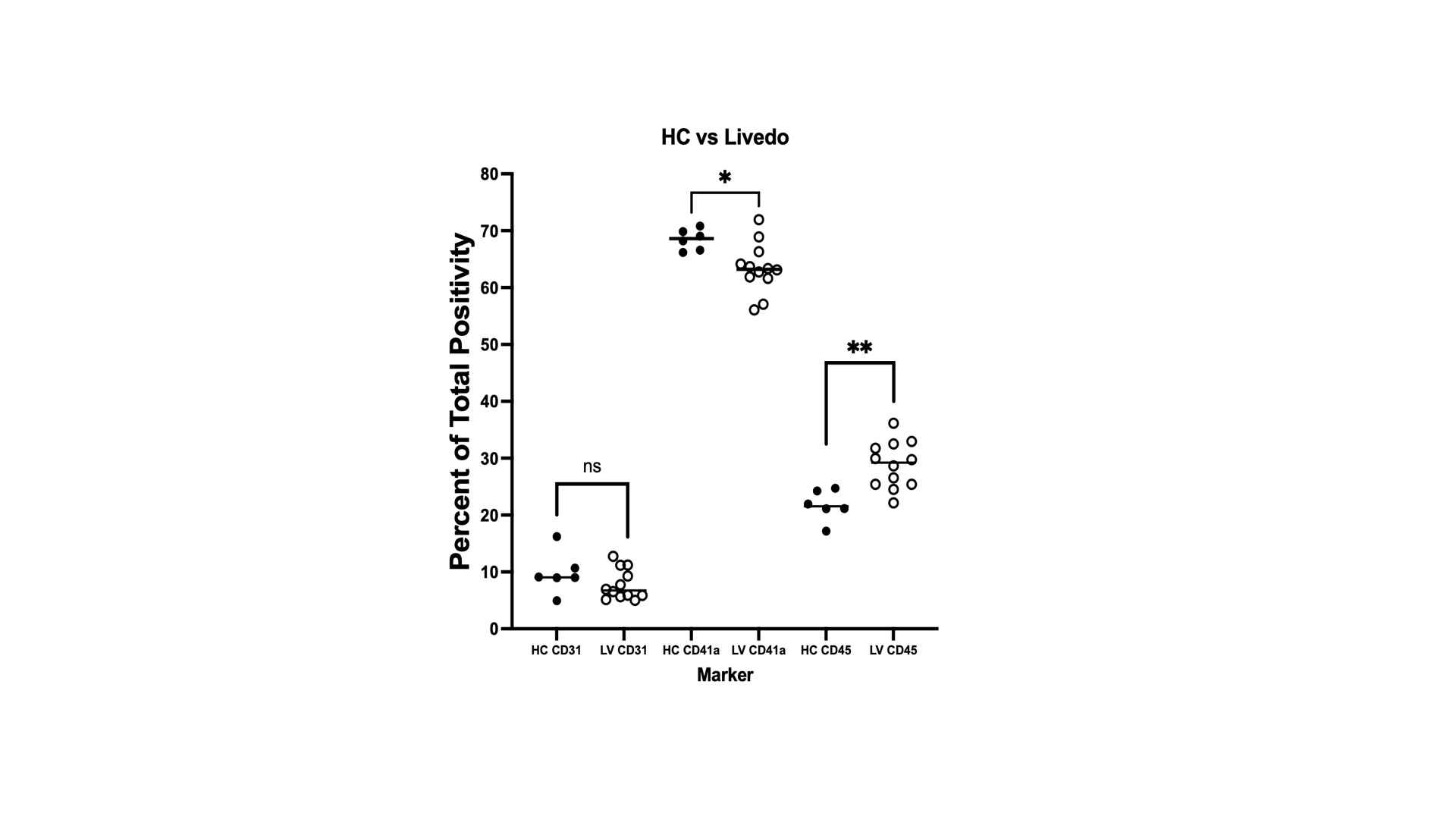
Results: TF procoagulant activity was increased in the plasma of LR patients compared to healthy controls (HCs). In EVs, we detected increased levels of TF activity in small EVs (SEV) but not in large EVs (LEV) in LR patients. In the process of sequential centrifugation, we measured TF activity in supernatants after each step. The 1st supernatant represented plasma without LEVs and the 2nd represented plasma depleted of both LEVs and SEVs. LR supernatants demonstrated a decrease in TF activity with each step, supporting a role for both large and small EVs in TF activation. This decrease was not seen in supernatants derived from HCs or from LPS-induced samples that were used as positive controls. We did not detect a difference in TF activity between large and small EVs. The concentration of vesicles in plasma was not increased in LR patients, implying the procoagulant properties of EVs may not be explained by a higher abundance, as reported in other procoagulant conditions, but possibly by their cargo. Hence, we sought to analyze the protein content of EVs. We detected 304 differentially expressed proteins (DEP) between LR and HCs. Five were upregulated and 269 downregulated. Most LR patients revealed a distinct expression pattern compared to HCs. A subset with profound vasculopathy showed a different pattern and for patients in remission the pattern was similar to HCs. Overrepresentation analysis revealed enrichment of proteins associated with homeostasis, coagulation, and vascular endothelial growth factor signaling pathways. Amongst the DEPs, Krueppel-Like Factor 8 (KLF8) exhibited significant prominence, demonstrating a substantial decrease in abundance by a fold change of 4.6 (p< 0.001). KLF8 is expressed in vascular endothelium and regulates angiogenesis and vessel contractility. Finally, we investigated the cells of origin of EVs in LR and controls previously identified as sources of procoagulant EVs. Our analysis revealed that patients with LR exhibited an increase in leukocyte-derived EVs and a decrease in platelet-derived EVs when compared with EVs derived from HCs. No significant changes were observed in the fraction of EVs that originated from endothelial cells. This may be indicative of a shift from baseline homeostasis in health to an inflammatory EV profile in LR.
Conclusion: EVs exert a procoagulant effect in LR. Increased TF activity and a shift in EV release from primarily platelet-driven to those originating from leukocytes are possible mechanisms underlying this effect. Our findings suggest that a reduction in KLF8 within EVs may be a key regulatory factor in LR.
To cite this abstract in AMA style:
Baniel A, Ricco C, Dhiman R, Eldaboush A, Stone C, Faden D, Musante L, Liu M, Werth V. Procoagulant Extracellular Vesicles in Patients with Livedo Reticularis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/procoagulant-extracellular-vesicles-in-patients-with-livedo-reticularis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/procoagulant-extracellular-vesicles-in-patients-with-livedo-reticularis/