Session Information
Date: Monday, November 14, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Baricitinib (BARI), an oral JAK1/JAK2 inhibitor, is in development for patients (pts) with moderate to severe RA.1,2 The purpose of this post hoc analysis of 2 completed Phase 3 studies was to determine if previous failure of cDMARDs altered the response to BARI in RA pts and to evaluate the effect of concomitant steroid use and prognostically unfavorable factors on the efficacy of BARI.
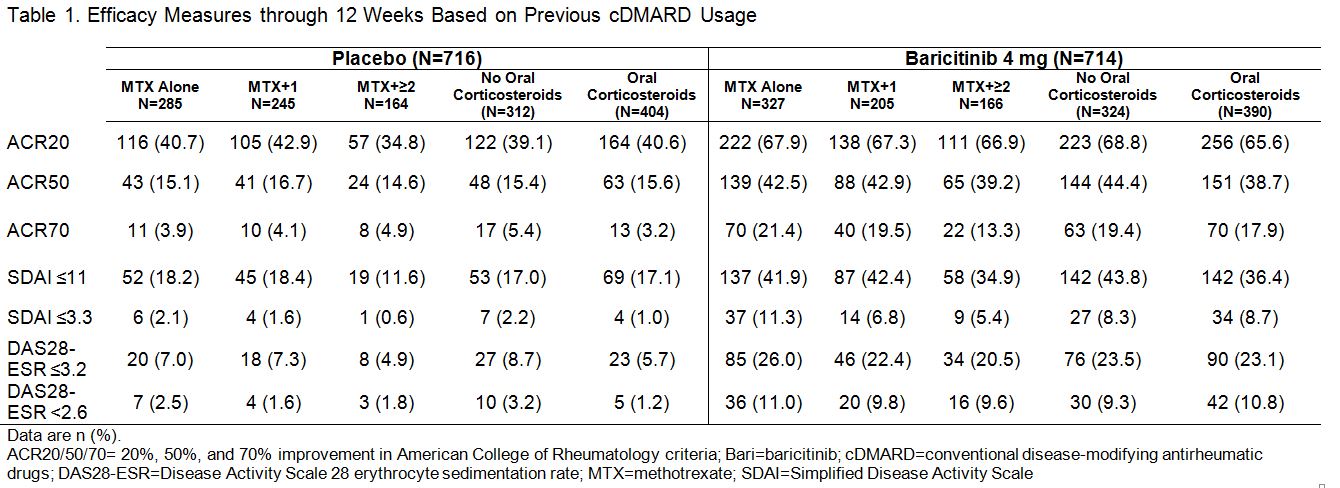
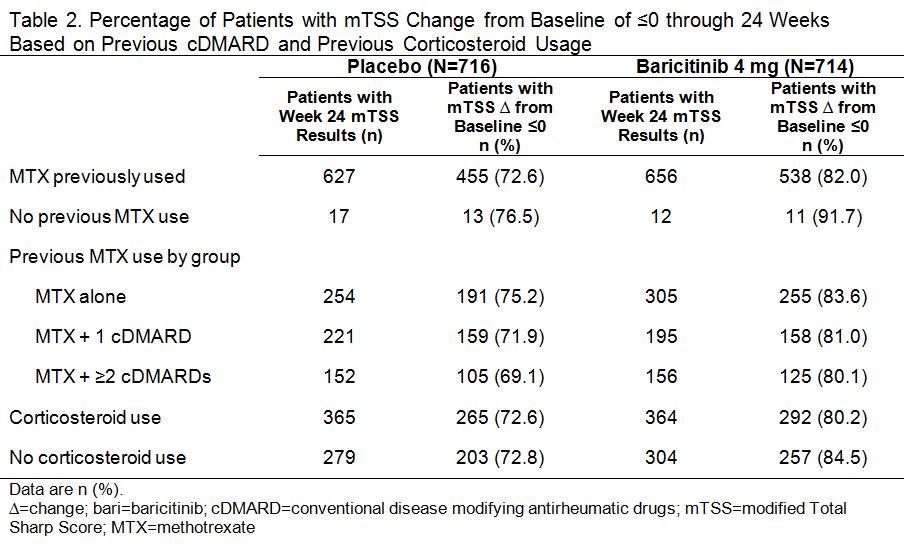
Methods: Pts with ≥6 swollen and tender joints and no prior biologic DMARD use were eligible for study inclusion. In RA-BUILD, cDMARD-inadequate responder (IR) pts with hsCRP ≥3.6 mg/L were randomized to placebo (PBO) or BARI (2 or 4 mg) once daily (QD).1 In RA-BEAM, MTX-IR pts with X-ray erosions and hsCRP ≥6.0 mg/L were randomized to PBO QD, BARI 4 mg QD, or adalimumab 40 mg biweekly.2 Patients continued background cDMARD (including MTX) therapy. The primary endpoint in both trials was ACR20 at Week 12 for BARI 4 mg vs. PBO.1,2 This post hoc analysis included the PBO (N=716) and BARI 4 mg (N=714) pts and assessed the number of previous cDMARDS, concurrent corticosteroid use, and effect of poor prognostic factors (high disease activity by Simplified Disease Activity Index [SDAI]>26), RF/ACPA positive, and radiographic erosions).
Results: In PBO pts, 40%, 34%, and 23% previously used MTX alone, MTX + 1 cDMARD, and MTX + ≥2 cDMARDs, respectively; in BARI 4 mg pts the rates were 46%, 29%, and 23%. Oral corticosteroids were used in 56% of PBO and 55% of BARI pts at baseline; pts continued use throughout the studies. Regardless of treatment assignment, the majority of pts had 2 or 3 of the prognostically unfavorable factors (95% in PBO; 96% in BARI pts). The primary objectives were met for both studies.1,2 The clinical efficacy of BARI 4 mg over PBO at 12 weeks (Table 1) and the percentage of pts with van der Heijde modified Total Sharp Score (mTSS) change from baseline ≤0 at 24 weeks (Table 2) each was similar regardless of the number of cDMARDs previously used, the concomitant use of corticosteroids, or the presence of prognostically unfavorable factors. The rates of serious adverse events and discontinuation due to adverse events were comparable regardless of the number of cDMARDs used, corticosteroid use, or the number of risk factors.
Conclusion: Baricitinib has demonstrated clinical efficacy in a wide range of patients with varying exposure to cDMARDs, concomitant use of corticosteroids, serologic status, and baseline disease activity. References: 1Dougados M et al. Ann Rheum Dis 2015;74(S2):79. 2Taylor PC et al. Arthritis Rheumatol 2015;67(S10):3927-3928.
To cite this abstract in AMA style:
Kavanaugh AF, van Vollenhoven RF, Muram D, Alam J, Arora V, de Macedo Pinto Correia AL, de la Torre I, O'Dell JR. Previous Use of Conventional Disease-Modifying Antirheumatic Drugs and Response to Baricitinib [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/previous-use-of-conventional-disease-modifying-antirheumatic-drugs-and-response-to-baricitinib/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/previous-use-of-conventional-disease-modifying-antirheumatic-drugs-and-response-to-baricitinib/