Session Information
Date: Tuesday, November 19, 2024
Title: Abstracts: RA – Treatment II: Refining Use of Established Therapies
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Currently, prevention possibilities of developing ACPA-negative rheumatoid arthritis (RA) are unknown. Studying this is challenging because many ACPA-negative at-risk individuals have low risk of RA; this predominance can conceal treatment effects in prevention trials. The TREAT EARLIER-trial included participants with ACPA-negative clinically suspect arthralgia (CSA). We hypothesized that studying efficacy in ACPA-negative CSA requires risk stratification. Therefore, we studied the efficacy of a 1-year course of methotrexate on reducing RA-development in ACPA-negative CSA after risk stratification, using TREAT EARLIER-data with 4-years follow-up.
Methods: This double-blind, placebo-controlled, proof-of-concept-trial in the southwest of the Netherlands, included 182 ACPA-negative and 54 ACPA-positive participants with CSA and subclinical joint inflammation. Participants were randomly assigned (1:1) to a single intramuscular glucocorticoid injection (120mg) and a 1-year course of oral methotrexate (up to 25mg/week) or placebo. Participants were stratified into low-risk (< 25% predicted risk for RA-development)/increased-risk (25-70%)/high-risk (≥70%). Primary endpoint RA-development was studied after 4-years. Treatment efficacy was determined per risk-group. Severity of subclinical joint inflammation, physical functioning and grip strength in ACPA-negative participants during 2-years was studied per risk-group. This trial is registered with EudraCT, 2014-004472-35, and the Netherlands Trial Register, NTR4853-trial-NL4599.
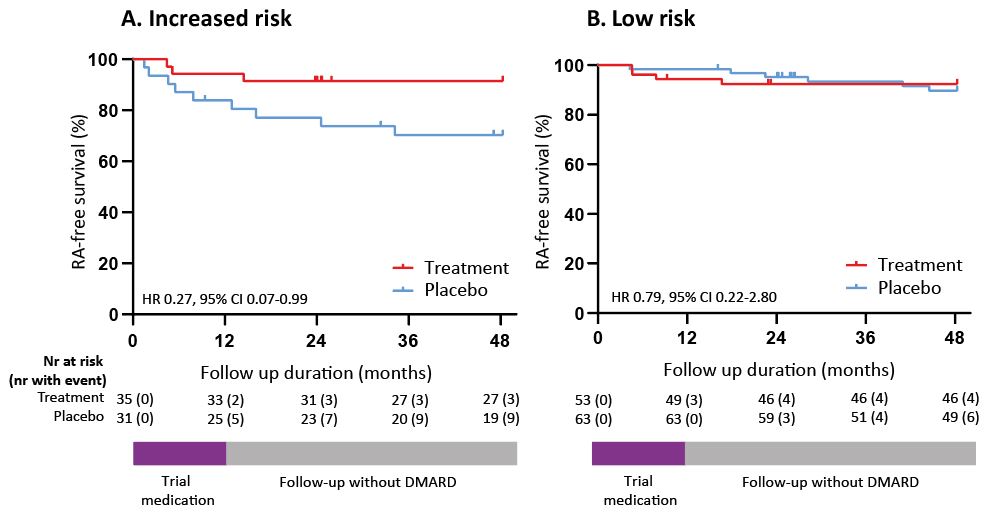
Results: 236 participants were included between April-2015 and September-2019 and randomized to active treatment (n=119) or placebo (n=117). 36% of ACPA-negative participants had predicted increased-risk and 64% low-risk. After 4-years in the ACPA-negative increased-risk group, 8.6% in the treatment-arm developed RA, compared to 29.0% in the placebo-arm (HR 0.27; 95%CI 0.07-0.99) (figure 1A). In ACPA-negative low-risk group, there was no difference in RA-development (7.5% vs 9.5%, HR 0.79; 95%CI 0.22-2.80) (figure 1B). Also, there was no sustained difference within ACPA-positive participants (58.1% vs 52.2%, HR 0.93; 95%CI 0.45-1.93). Subclinical joint inflammation, physical functioning and grip strength persistently improved upon treatment in ACPA-negative participants with increased-risk, but not in those with low-risk.
Conclusion: This is the first evidence to suggest that the development of ACPA-negative RA can be prevented by a 1-year course of methotrexate in CSA.
To cite this abstract in AMA style:
Dumoulin Q, Krijbolder D, Visser K, Lard L, van der Helm-van Mil A. Prevention of the Development of Rheumatoid Arthritis by a 1-year Course of Methotrexate in ACPA-negative Arthralgia Patients at Increased Risk for Rheumatoid Arthritis: 4 Year Results of the TREAT EARLIER Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/prevention-of-the-development-of-rheumatoid-arthritis-by-a-1-year-course-of-methotrexate-in-acpa-negative-arthralgia-patients-at-increased-risk-for-rheumatoid-arthritis-4-year-results-of-the-treat-ea/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prevention-of-the-development-of-rheumatoid-arthritis-by-a-1-year-course-of-methotrexate-in-acpa-negative-arthralgia-patients-at-increased-risk-for-rheumatoid-arthritis-4-year-results-of-the-treat-ea/