Session Information
Date: Sunday, October 26, 2025
Title: (0593–0640) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is an autoimmune disease associated with an increased risk of chronic kidney disease (CKD), avascular necrosis (AVN), and mortality. Despite advancements in management, the contemporary prevalence and risk factors for these complications remain incompletely characterized. This study aimed to assess these outcomes within the context of modern SLE management.
Methods: We conducted a retrospective analysis of patients with SLE who received follow-up care at a tertiary referral center between April 2006 and February 2023. The prevalence and risk factors for lupus nephritis, progression to CKD stage 4 or 5, AVN, and all-cause mortality were evaluated using hazard ratios.
Results: Among 484 patients, lupus nephritis was observed in 26.2%, AVN in 4.8%, and CKD stage 4 or 5 in 2.9%, while the overall mortality rate was 2.5%. Lupus nephritis was more prevalent in patients diagnosed before age 20, whereas CKD progression and mortality were significantly higher in those with later-onset SLE. Hydroxychloroquine use was protective against CKD progression (HR 0.19, 95% CI 0.05–0.69, p = 0.012) and mortality (HR 0.14, 95% CI 0.031–0.66, p = 0.013). Methylprednisolone pulse therapy was associated with an increased risk of AVN (HR 6.10, 95% CI 2.42–15.35, p < 0.001).
Conclusion: Compared to historical cohorts, the prevalence of CKD stage 4 or 5, AVN, and mortality has declined in this cohort. Hydroxychloroquine improves outcomes by reducing CKD progression and mortality, while caution is warranted with methylprednisolone pulse therapy due to its association with AVN.
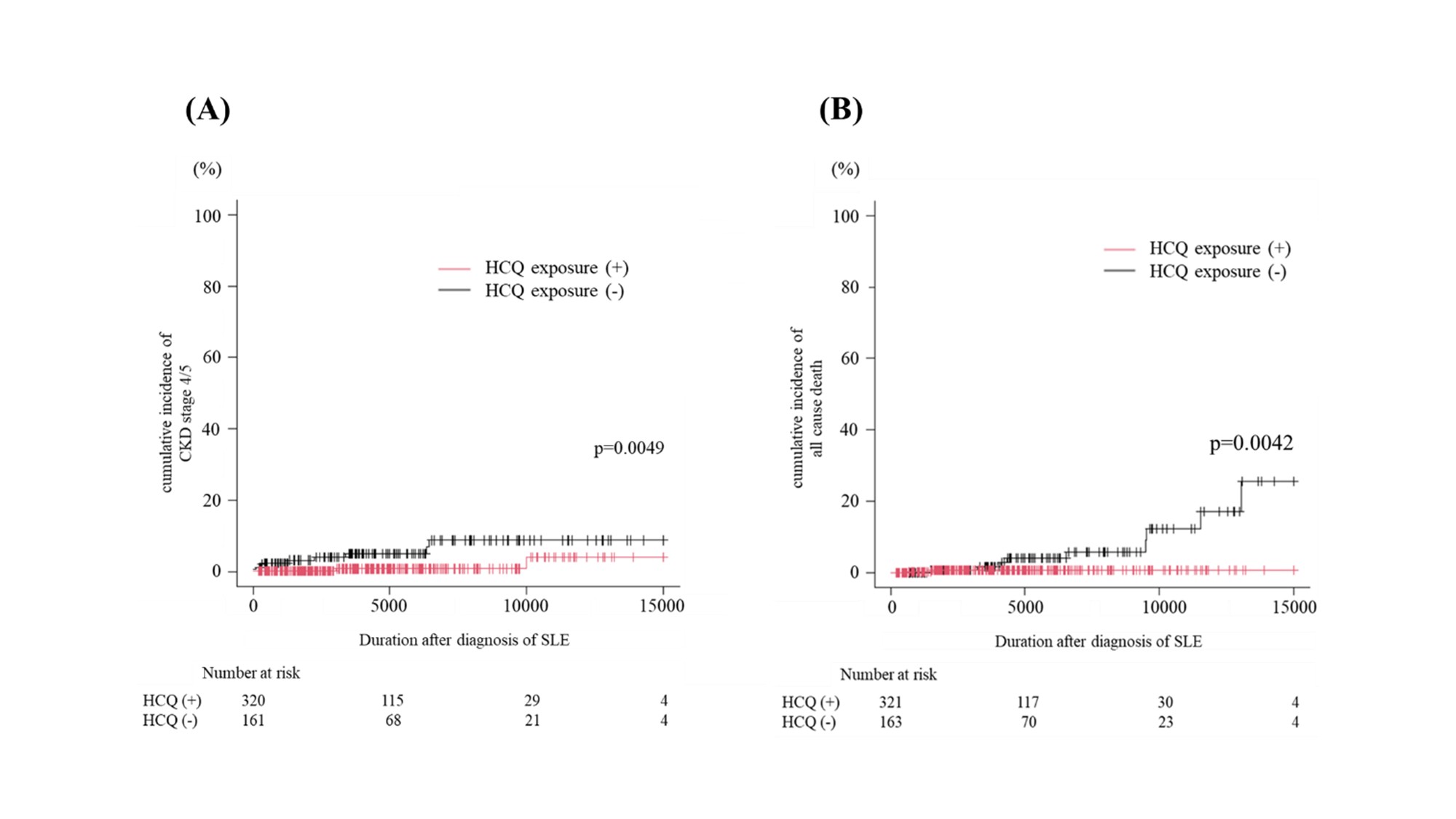 Figure 1 Hydroxychloroquine prescription and its impact on cumulative incidence of CKD stage 4 or 5 and all-cause mortality
Figure 1 Hydroxychloroquine prescription and its impact on cumulative incidence of CKD stage 4 or 5 and all-cause mortality
(A) Hydroxychloroquine prescription and its impact on cumulative incidence of CKD stage 4 or 5
(B) Hydroxychloroquine prescription and its impact on cumulative incidence of all-cause mortality
.jpg) Table 1: Hazard ratio of incidence of CKD stage 4 or 5
Table 1: Hazard ratio of incidence of CKD stage 4 or 5
.jpg) Table 2: Hazard ratio of incidence of all cause death
Table 2: Hazard ratio of incidence of all cause death
To cite this abstract in AMA style:
Nakai T, Fukui S, Ikeda Y, Suda M, Tamaki H, kishimoto m, Okada M. Prevalence of lupus nephritis, end stage kidney disease, avascular necrosis, and mortality in systemic lupus erythematosus in the recent era [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/prevalence-of-lupus-nephritis-end-stage-kidney-disease-avascular-necrosis-and-mortality-in-systemic-lupus-erythematosus-in-the-recent-era/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prevalence-of-lupus-nephritis-end-stage-kidney-disease-avascular-necrosis-and-mortality-in-systemic-lupus-erythematosus-in-the-recent-era/
