Session Information
Title: Epidemiology and Public Health (ACR): Rheumatoid Arthritis and Systemic Lupus Erythematosus Outcomes
Session Type: Abstract Submissions (ACR)
Background/Purpose: Little is known about the trends of medication use in pregnant women with autoimmune disorders. The objective of the current study was to examine the prevalence and trends of oral steroids and immunomodulatory drug use in pregnant women with rheumatoid arthritis (RA), psoriasis, and systemic lupus erythematosus (SLE).
Methods: A cohort of pregnant women with RA, psoriasis, or SLE was identified using data from the Medicaid Analytical eXtract for the period of 2000-2007. The following 3 classes of medications were identified using prescription dispensing data for these patients; 1) oral steroids, 2) non-biologic disease modifying anti-rheumatic drugs (nbDMARDs), and 3) biologic DMARDs. The proportion of women with RA, psoriasis, and SLE exposed to these medications in the following time-windows were reported, 1) 3 months pre-last menstrual period (LMP), 2) first trimester, 3) second trimester, and 4) third trimester. Trends in the use were also evaluated by calendar year.
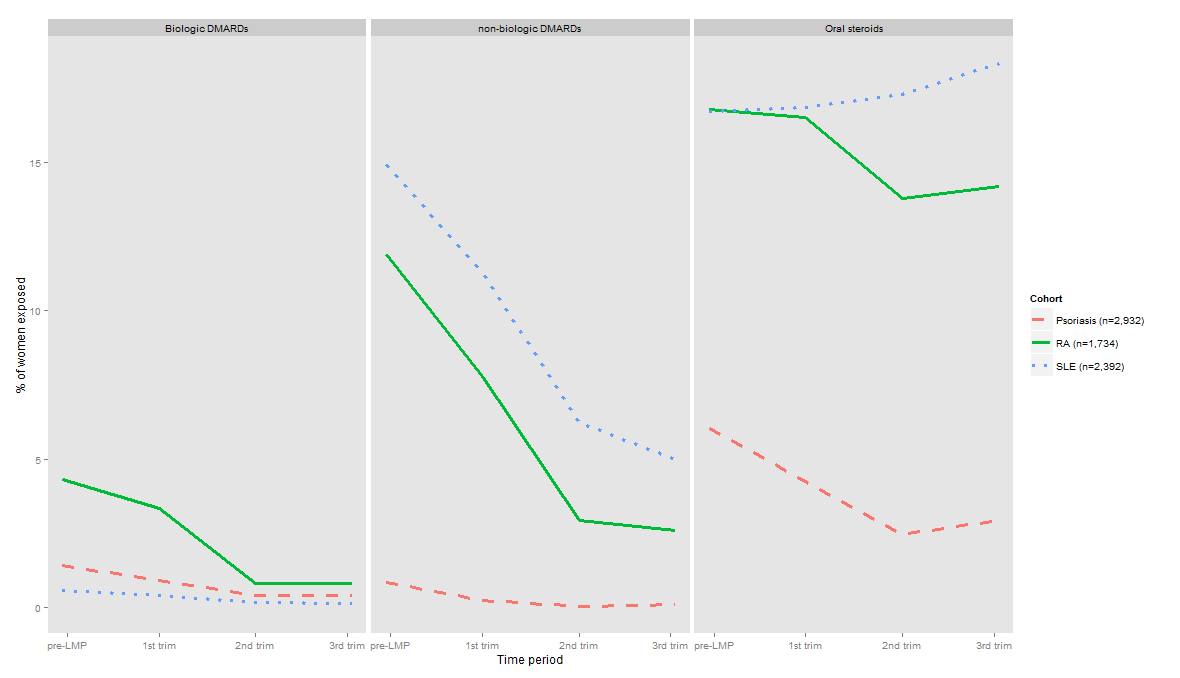
Results: A total of 1,734 pregnant women with RA, 2,932 with psoriasis and 2,392 with SLE enrolled in Medicaid from 46 states and Washington, DC were identified. During the 3 months pre-LMP, 16.8% of women with RA used oral steroids, 11.9% used nbDMARDs, and 4.3% used biologics. During pregnancy, the use of steroids showed modest reduction in women with RA compared to 3 months pre-LMP use (Figure). However, the use of nbDMARDs showed a marked reduction during the 1st, 2nd and 3rd trimesters with 7.8%, 2.9%, and 2.6% of women with RA using these agents during these periods, respectively. Similarly, the use of biologics in women with RA also decreased during pregnancy. In women with psoriasis, 6.3% used oral steroids, 0.8% used nbDMARDs, and 1.4% used biologics during the 3 months pre-LMP period. During pregnancy, use decreased for all the three classes of medications. In women with SLE, 16.7% used oral steroids, 14.9% used nbDMARDs, and 0.6% used biologics during the 3 months pre-LMP. Use of steroids was increased moderately during pregnancy compared to 3 months pre-LMP use, but a lower proportion of women used non-biologic and biologics during pregnancy in the SLE cohort. No meaningful time trends were observed in use of these three classes of medications during pregnancy between 2000 and 2007.
Conclusion: We observed reduced use of non-biologic and biologics in women with RA, psoriasis, and SLE during pregnancy compared to before pregnancy. This reduced use may be due to lowered disease activity during pregnancy or fetal safety concerns related to the use of these agents. Oral steroids were the most commonly used therapy in all three populations at all the stages of pregnancy. Given this high use, future research evaluating the safety of steroids in pregnancy is warranted.
Figure: Trends in use of oral steroids, non-biologic and biologic DMARDs at different stages of pregnancy in women with RA, psoriasis, and SLE
Disclosure:
R. Desai,
Biogen Idec,
1;
K. Huybrechts,
None;
B. Bateman,
None;
H. Mogun,
None;
S. Hernandez-diaz,
Novartis, GSK, AstraZeneca,
5;
S. C. Kim,
Pfizer Inc,
2.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prescription-medication-trends-in-medicaid-enrolled-pregnant-women-with-rheumatoid-arthritis-psoriasis-and-systemic-lupus-erythematosus/