Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: About 50% of children with Juvenile Idiopathic Arthritis (JIA) receiving methotrexate (MTX) develop MTX intolerance with severe anticipatory nausea/vomiting and avoidance behaviors. Intolerance often leads to poor adherence or stopping MTX. Our aim was to evaluate if routine premedication with the anti-emetic ondansetron reduces MTX intolerance and increases the proportion of children able to continue MTX, compared to as-needed ondansetron use.
Methods: Children 4-16 years in the Canadian Alliance of Pediatric Rheumatology Investigators (CAPRI) Registry who were starting MTX for the first time were assigned, via stratified block randomization by Canadian region and patient weight, to: 1) routine premedication with oral ondansetron from the very first dose of MTX (intervention), or 2) ondansetron used only after developing nausea/vomiting (control). Ondansetron was prescribed as 3 doses weekly starting one hour before MTX (2mg if < 15Kg, 4 mg if 15-30 Kg, or 8mg if >30Kg). The primary endpoint was the proportion of children who remain on MTX with good tolerance one year after starting MTX. We hypothesized this would happen in ≥75% intervention patients versus 50% in control patients. Intolerance was ≥6 points in the MTX Intolerance Severity Score. The COVID19 pandemic slowed recruitment and the planned interim analysis was conducted with patients recruited as of October 6, 2023. Any patient who developed intolerance or discontinued MTX within a year of starting it was considered a failure, and one-year success rates were estimated with Kaplan Meier survival analysis.
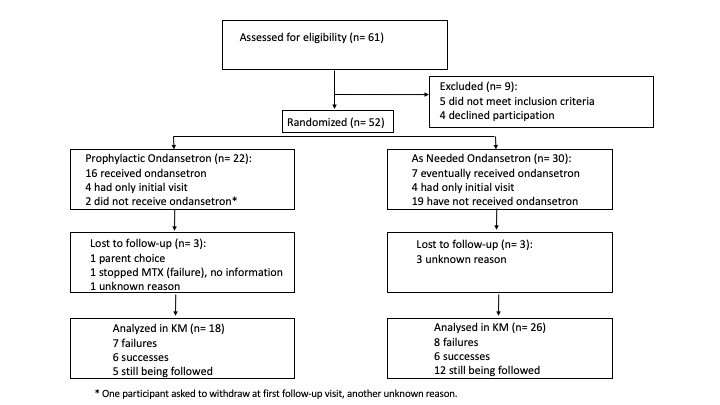
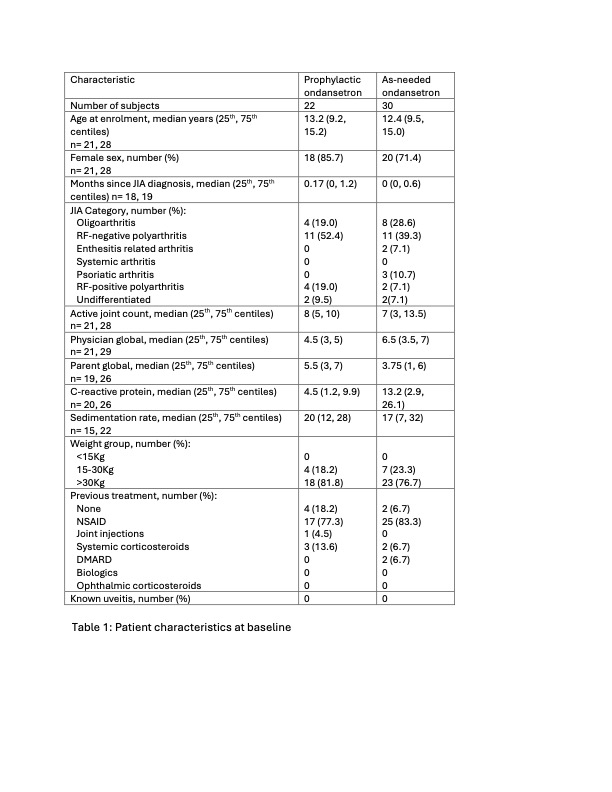
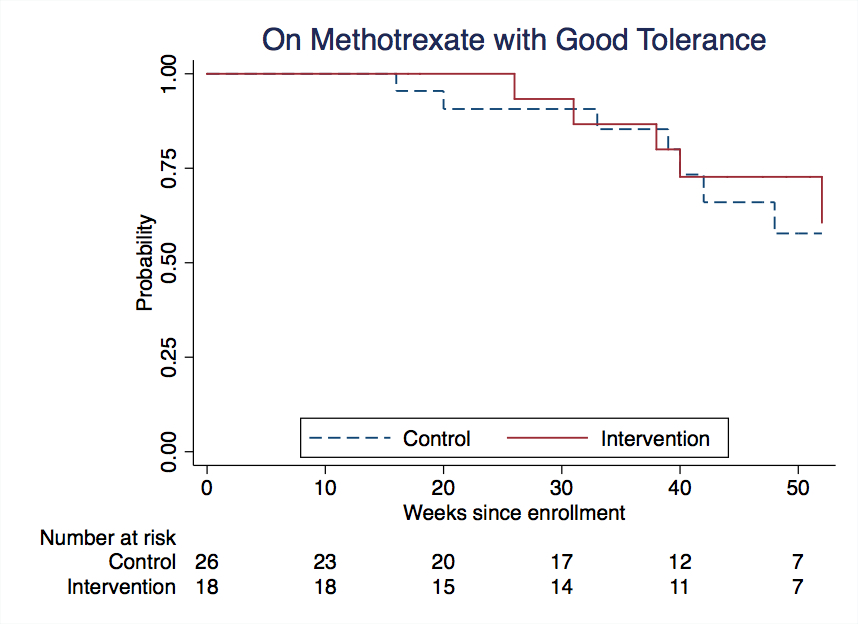
Results: Sixty-one children were assessed for eligibility, 52 were randomized and 44 contributed to the Kaplan Meier analysis. Participants randomized to intervention (n=22) or control (n=30) had comparable baseline characteristics, despite the imbalance in number of participants due to use of stratified blocks. After a median follow-up of 45.5 weeks (intervention) and 39 weeks (controls), we observed 7 failures in the intervention group and 8 failures in the control group with 59% and 83% of participants still on MTX at last follow-up, respectively. The estimated success at one year was 57% for intervention and 59% for controls (risk ratio 0.91; 99% CI 0.39, 2.08). Based on computer simulations, the probability of observing results like this if our a hypothesized success rates (75% and 50%, respectively) are correct is 0.055. Forty-six adverse events were reported (mostly gastrointestinal, 17 in intervention and 29 in control, respectively), and one was serious (infection with hospitalization in a patient not receiving ondansetron).
Conclusion: This interim analysis showed very little signal to suggest prophylactic ondansetron increases the proportion of patients remaining on MTX with no intolerance one year after starting it, relative to using ondansetron after nausea or vomiting develop. Based on these results, the Data and Safety Committee deemed enrolling additional patients was unlikely to change findings. It recommended stopping recruitment into the trial and completing follow-up of all subjects already enrolled.
NCT04169828. Funded by The Arthritis Society, Canada.
To cite this abstract in AMA style:
Chedeville G, Schmeling H, Proulx-Gauthier J, Batthish M, De Bruycker J, Feldman B, Berard R, Bolaria R, Chhabra A, Lim L, Huber A, Berkowitz M, Loughin T, Guzman J. Preliminary Results of the Ondansetron Pre-medication Trial in Juvenile Idiopathic Arthritis: A Pragmatic Randomized Controlled Trial Nested in the CAPRI Registry [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/preliminary-results-of-the-ondansetron-pre-medication-trial-in-juvenile-idiopathic-arthritis-a-pragmatic-randomized-controlled-trial-nested-in-the-capri-registry/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/preliminary-results-of-the-ondansetron-pre-medication-trial-in-juvenile-idiopathic-arthritis-a-pragmatic-randomized-controlled-trial-nested-in-the-capri-registry/