Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Methods:
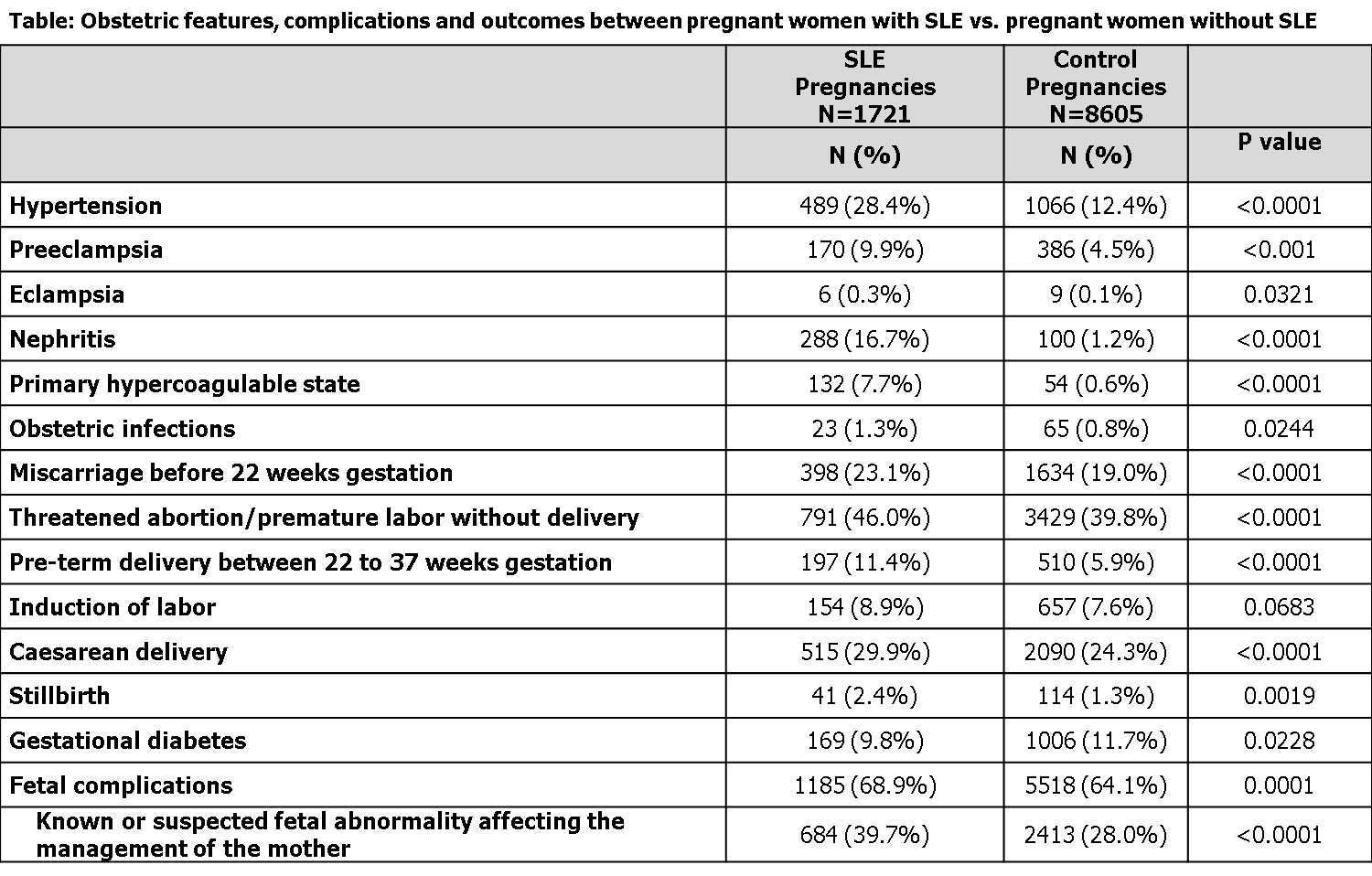
Results:
Conclusion:
In this large database analysis of over 10,000 pregnant women, those with SLE had a higher risk for suspected fetal abnormalities affecting care of the mother, and a doubling of risk for hypertension, preeclampsia, pre-term delivery and stillbirths. However, increased risk for earlier adverse pregnancy events, including miscarriage prior to 22 weeks and threatened abortion, appeared modest. These data highlight the need for regular physician-patient interaction, counseling and high quality education for women with SLE who are pregnant or looking to conceive to ensure possible complications are recognized early and managed appropriately.
Disclosure:
M. A. Petri,
UCB Pharma,
2,
UCB Pharma,
5;
P. Daly,
None;
D. S. Pushparajah,
UCB Pharma,
3;
D. Friesen,
UCB Pharma,
3;
L. Makaroff,
UCB Pharma,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pregnancy-complications-in-lupus-retrospective-observational-analysis-from-a-us-health-claims-database/