Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Numerous indicators have been proposed to evaluate the efficacy for randomized clinical trials (RCTs) of psoriasis (Pso) and psoriatic arthritis (PsA), but the comparability and correlation remain unknown. To evaluate the preference and relative sensitivity of the mostly widely used indicators that report response rate, and to provide advice for the primary endpoint selection for Pso and PsA.
Methods: We conducted a systematic search including 3 databases and 4 registries to identify all pharmacological intervention-controlled RCTs of Pso. Bayesian hierarchical linear mixed model assessed the relative discriminations and provided a ranking of these indicators.
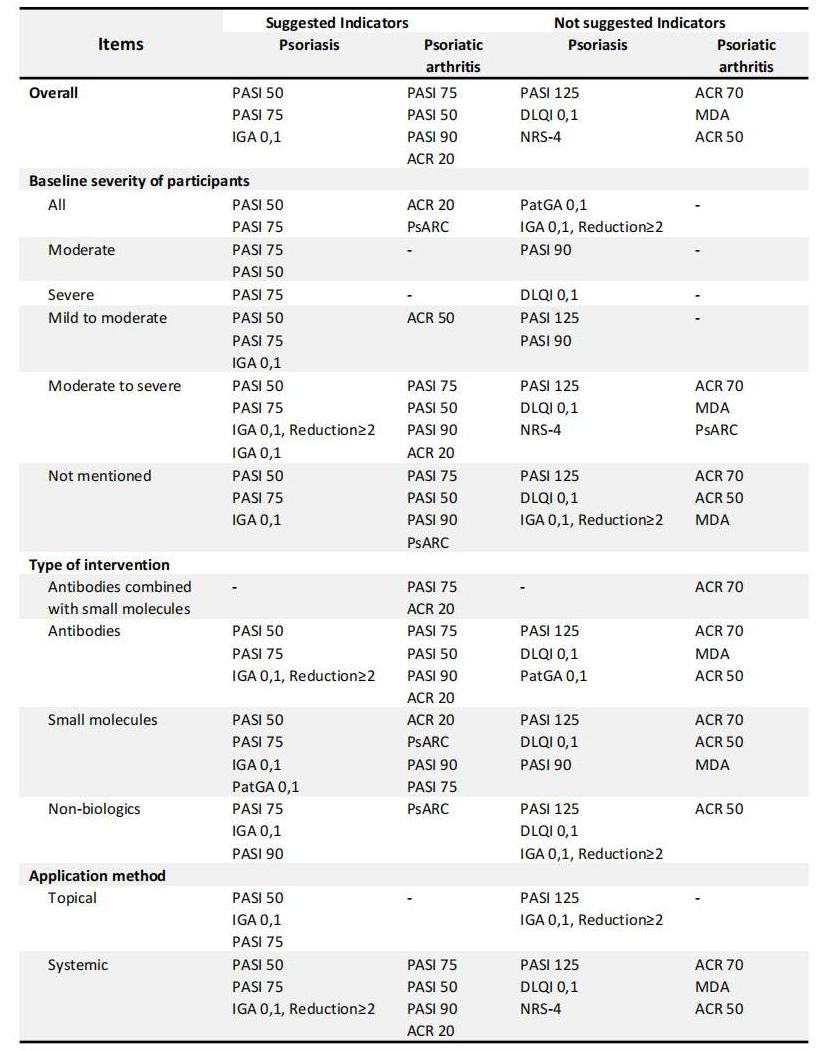
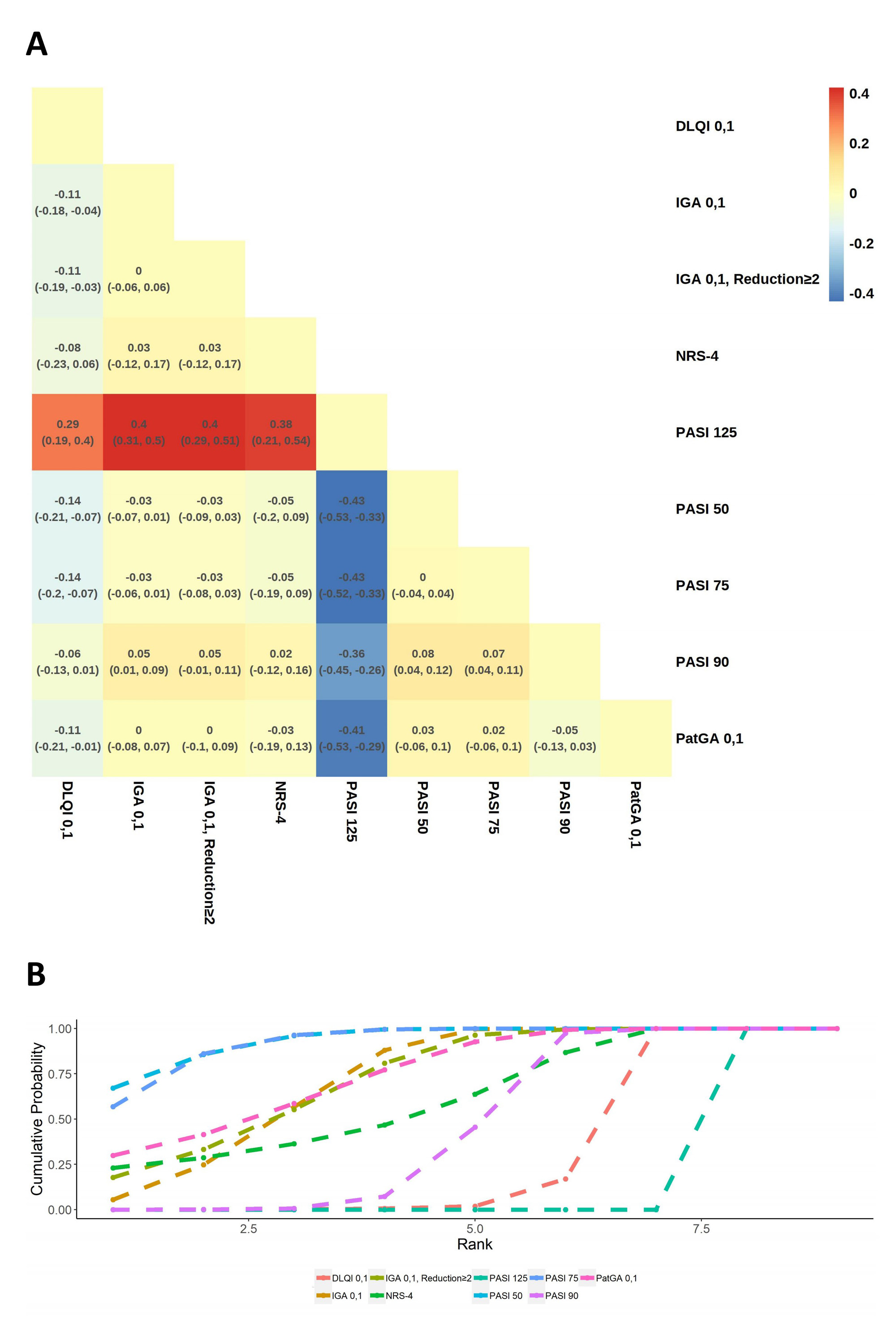
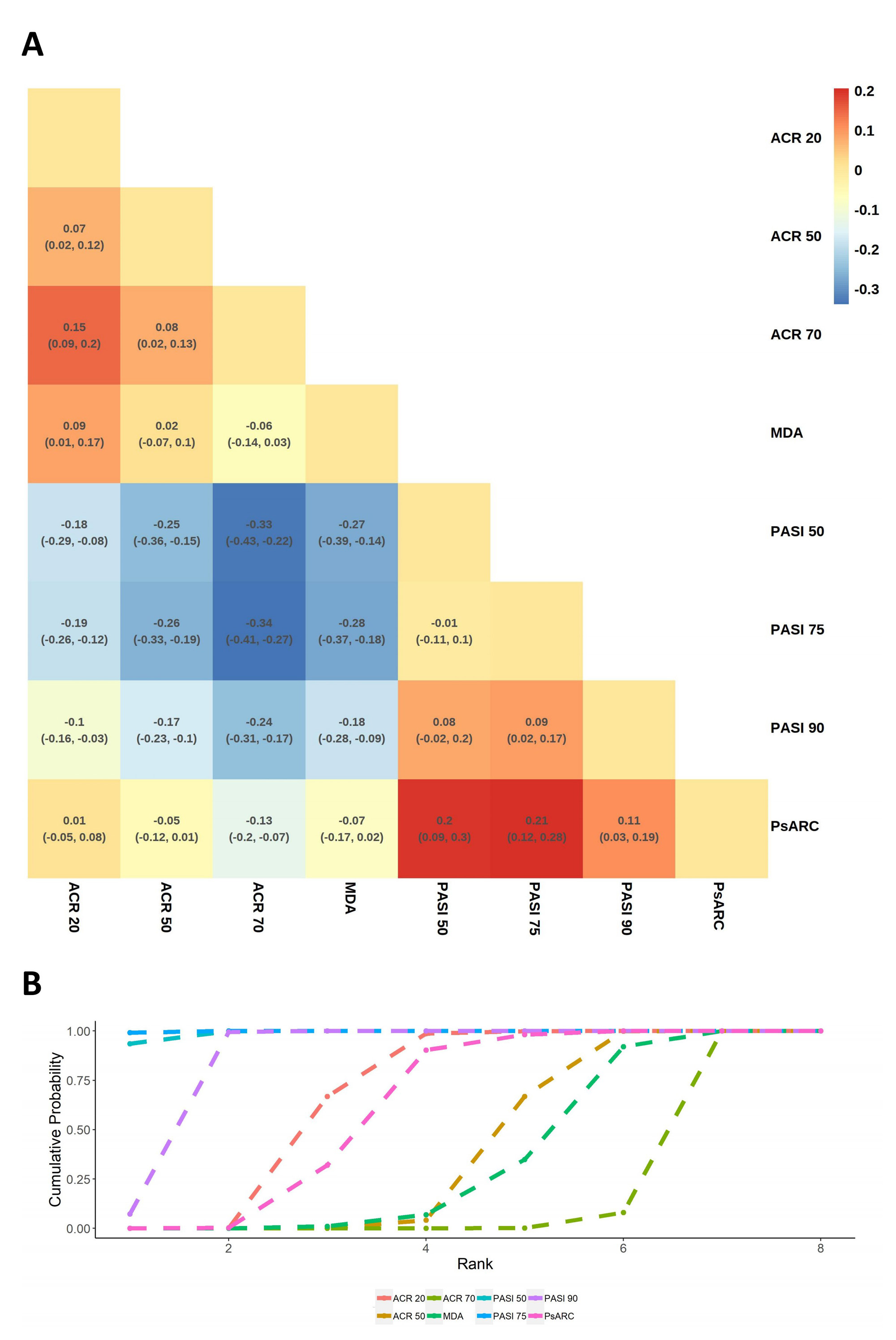
Results: Altogether 326 RCTs met our inclusion criteria, with 285 and 65 records on Pso and PsA, respectively. We found evidence of significant differences between indicators. Psoriasis Area and Severity Index (PASI) 50, PASI 75 and Investigator’s Global Assessment (IGA) 0,1 were powerful indicators to reveal the pharmacological efficacy in most RCTs of Pso(Figure 1). In contrast, PASI 125, Dermatology Life Quality Index (DIQI) 0,1 and Numerical Rating Scale (NRS) 4 were not preferred under different circumstances. Additionally, PASI 50, PASI 75 and PASI 90 seemed to be the most effective in nearly all types of pharmacological RCTs of PsA (Figure 2). However, due to their extremely sensitivity, American College of Rheumatology (ACR) 20 was also recommended to avoid exaggerating the therapeutic advantages of interventions. Instead, ACR 50, ACR 70 and Minimal Disease Activity (MDA) were the least sensitive, but they were supposed to be more cautious in evaluating disease changing. Indicator preference was slightly altered by disease severity, intervention type and administration method(Table 1).
Conclusion: The impressionable efficacy discrimination ability of indicators highlights the importance of flexibility and comprehensiveness when choosing primary outcome(s). As for trials that are only evaluated by indicators with extremely sensitivity, attention should be paid to the outcome interpretation to avoid the exaggeration of treatment efficacy.
To cite this abstract in AMA style:
Tian J, Kang S, Zhang D, Lu Q. Preference and Relative Sensitivity of Indicators Reporting Response Rate in Pharmaceutical Trials of Psoriasis and Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/preference-and-relative-sensitivity-of-indicators-reporting-response-rate-in-pharmaceutical-trials-of-psoriasis-and-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/preference-and-relative-sensitivity-of-indicators-reporting-response-rate-in-pharmaceutical-trials-of-psoriasis-and-psoriatic-arthritis/