Session Information
Date: Monday, November 8, 2021
Title: RA – Treatments Poster II: PROs, Biomarkers, & Systemic Inflammation (1223–1256)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Early prediction of response to treatment with upadacitinib (UPA) 15 mg once daily (QD) could help optimize therapy in patients (pts) with RA.1-4 The objective of this analysis was to identify baseline (BL) characteristics or Week (Wk) 12 disease activity measures that may predict the achievement of remission (REM) or low disease activity (LDA) at 6 months in pts with RA.
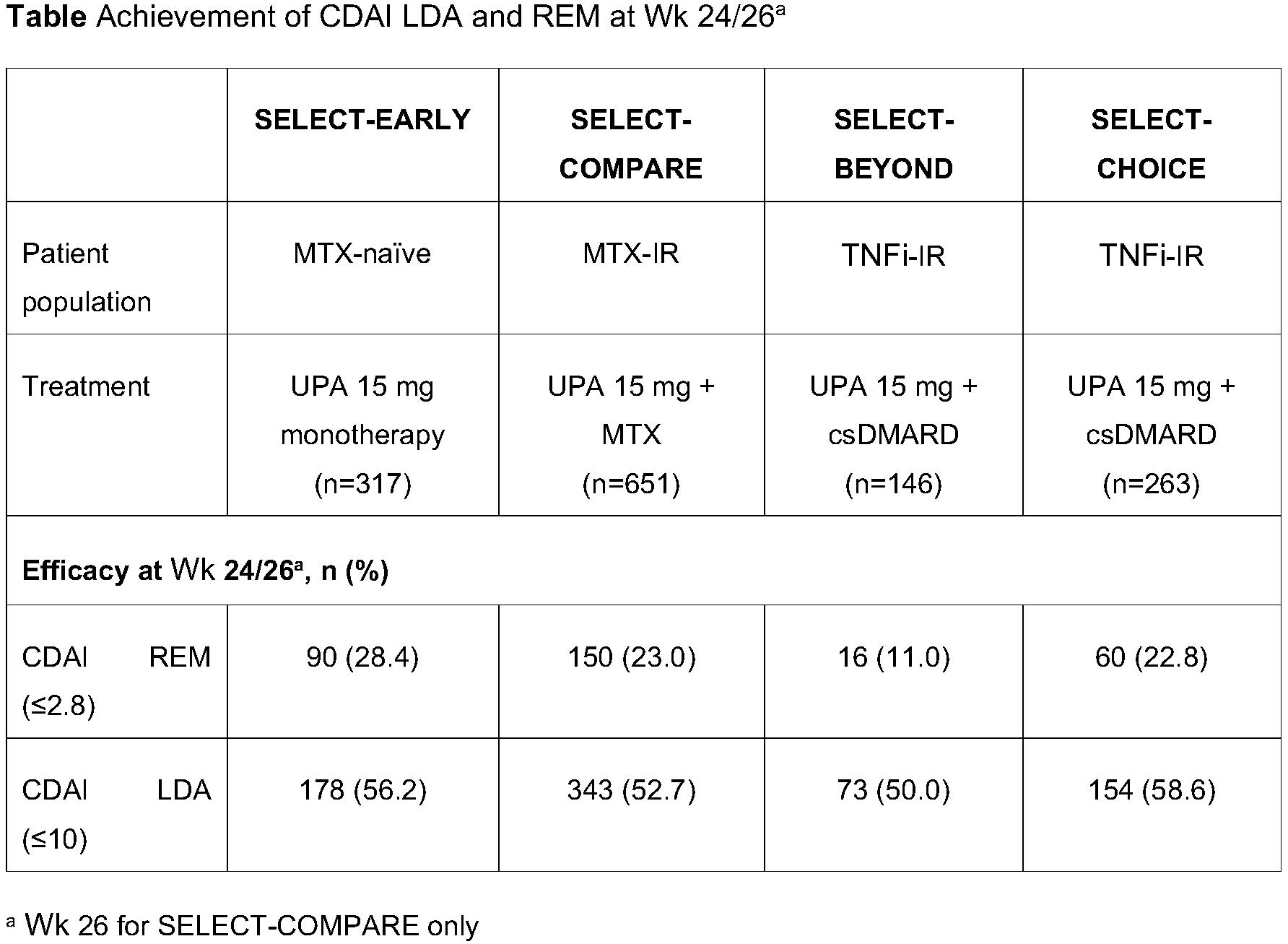
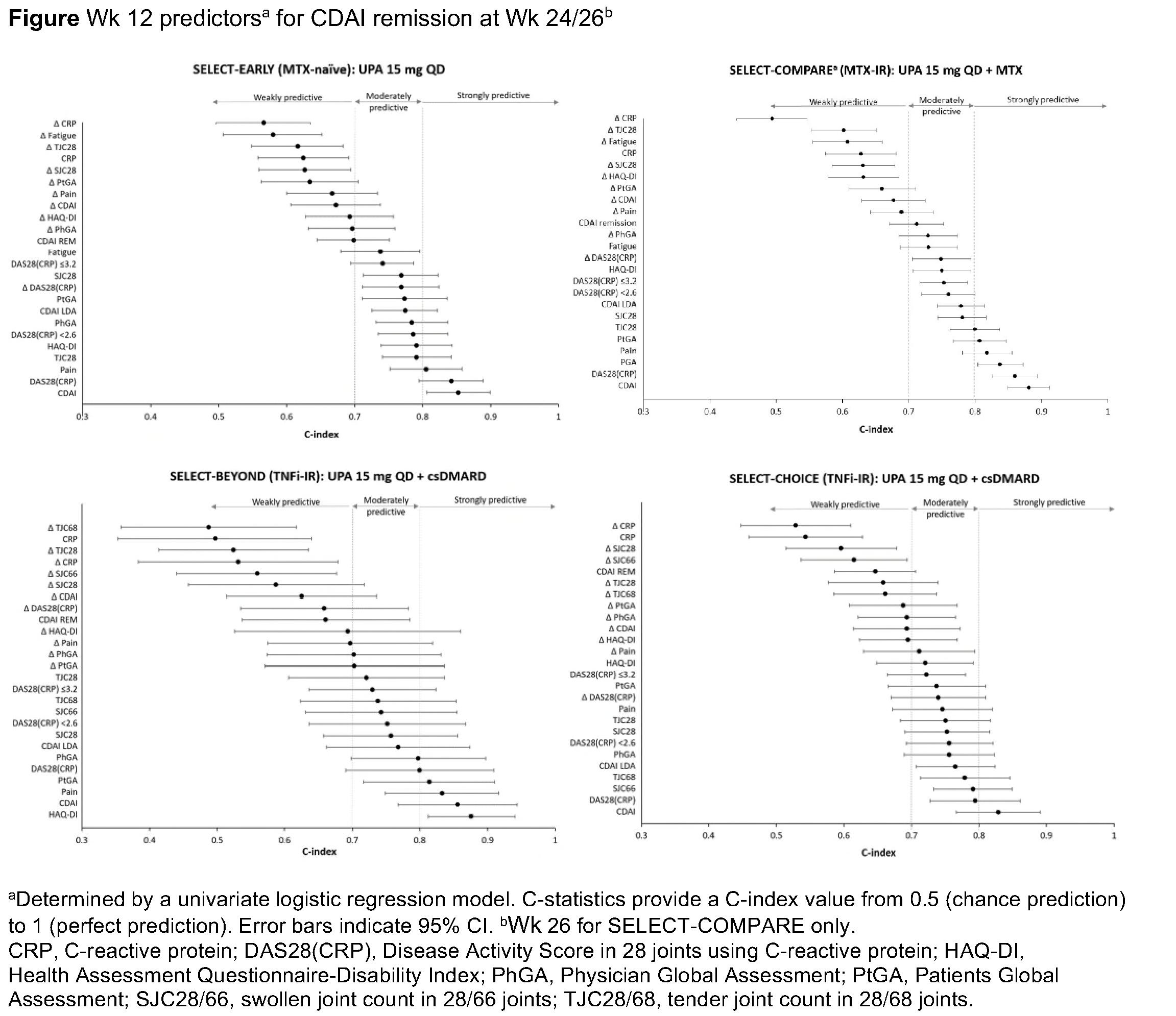
Methods: This ad hoc analysis included pts randomized to UPA 15 mg QD, as monotherapy in MTX-naïve pts (SELECT-EARLY) or in combination with conventional synthetic DMARDs (csDMARDs), in pts with inadequate response (IR) to MTX (SELECT-COMPARE) or ≥1 tumor necrosis factor inhibitors (TNFis) (SELECT-BEYOND and SELECT-CHOICE). Association of BL characteristics (age, disease duration, prior/concomitant treatments, C-reactive protein [CRP], seropositivity, and disease activity) and Wk 12 disease activity parameters with achievement of Clinical Disease Activity Index (CDAI) REM (≤2.8) or LDA (≤10) at Wk 24 (or Wk 26 in SELECT-COMPARE) was assessed by concordance statistics (C-statistics), or area under the receiver operator characteristic curve. C-index values and 95% confidence intervals were calculated by fitting a univariate logistic regression model for: demographic and BL characteristics, Wk 12 disease activity measures, and change from BL at Wk 12 in disease activity measures. A multivariate logistic regression with stepwise model selection was also performed. Proportion of pts achieving Wk 24/26 CDAI REM/LDA was stratified by ≥50% improvement from BL in swollen and/or tender joint count in 66/68 joints (SJC66/TJC68).
Results: A total of 1377 pts were included. Across the 4 studies, CDAI REM and LDA were achieved in 11.0–28.4% and 50.0–58.6% of pts, respectively (Table). BL demographics and disease characteristics were weakly predictive (C-index < 0.70) of Wk 24/26 CDAI REM (C-index 0.49–0.69) or LDA (C-index 0.47–0.65), except for BL disability index of HAQ (HAQ-DI) in SELECT-BEYOND, which was moderately predictive of CDAI REM (C-index 0.73). Changes from BL in disease activity measures at Wk 12 were weakly or moderately predictive of Wk 24/26 CDAI REM (Figure) or LDA. CDAI value at Wk 12 was strongly predictive (C-index >0.80) of Wk 24/26 CDAI REM or LDA. Disease Activity Score in 28 joints using CRP and pain at Wk 12 were strongly predictive of Wk 24/26 CDAI REM (except in SELECT-CHOICE). Physician’s global assessment at Wk 12 was the only common predictor in multivariate regression models for CDAI REM/LDA at Wk 24/26 across studies. Greater proportion of pts achieving ≥50% improvement in SJC66 and TJC68 at Wk 12 achieved CDAI REM (16.5–37.8% vs 0–9.4%) or LDA (66.0–72.8% vs 20.9–35.7%) at Wk 24/26 than those who did not.
Conclusion: BL characteristics did not strongly predict response to UPA, but composite disease activity scores at Wk 12 predicted Wk 24/26 REM/LDA with UPA 15 mg QD across MTX-naïve, MTX-IR, and TNFi-IR pts. ≥50% improvement in SJC/TJC at Wk 12 was also associated with Wk 24/26 REM/LDA.
References:
1. van Vollenhoven R, et al. Arthritis Rheumatol 2020;72:1607–20.
2. Genovese MC, et al. Lancet 2018;391:2513–24.
3. Fleischmann R, et al. Arthritis Rheumatol 2019;71:1788–800.
4. Rubbert-Roth A, et al. N Engl J Med 2020;383:1511–21.
To cite this abstract in AMA style:
Kavanaugh A, Szekanecz Z, Keystone E, Rubbert-Roth A, Hall S, Xavier R, Pereira J, Song I, Martin N, Song Y, Anyanwu S, Nash P. Predictors of Response: Baseline Characteristics and Early Treatment Responses Associated with Achievement of Remission and Low Disease Activity Among Upadacitinib-Treated Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/predictors-of-response-baseline-characteristics-and-early-treatment-responses-associated-with-achievement-of-remission-and-low-disease-activity-among-upadacitinib-treated-patients-with-rheumatoid-art/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-of-response-baseline-characteristics-and-early-treatment-responses-associated-with-achievement-of-remission-and-low-disease-activity-among-upadacitinib-treated-patients-with-rheumatoid-art/