Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The SENSCIS trial and the SENSCIS-ON study have shown that nintedanib (NTD) reduces the rate of decline in Interstitial Lung Disease (ILD) in Systemic Sclerosis (SSc) patients. We aimed to analyse the predictors of response and NTD-related side effects at 1-year and efficacy and safety of NTD at 2-year in SSc-ILD in a real-life setting.
Methods: The clinical data of SSc-ILD patients treated with NTD from 13 Italian SSc centres were retrospectively evaluated 12 months prior to NTD introduction, at baseline, and at 12 and 24 months after NTD introduction. The following parameters were recorded: SSc clinical features, concomitant therapies, NTD tolerability, pulmonary function tests (PFTs) and modified Rodnan skin score (mRSS). Progression was defined according to ATS definition (drop in predicted% FVC³5% or DLCO³10% or high-resolution chest tomography (HRCT) evolution and worsening of respiratory symptoms). Logistic regression analyses were performed to assess predictors of response at 1-year and predictors of NTD reduction/suspension.
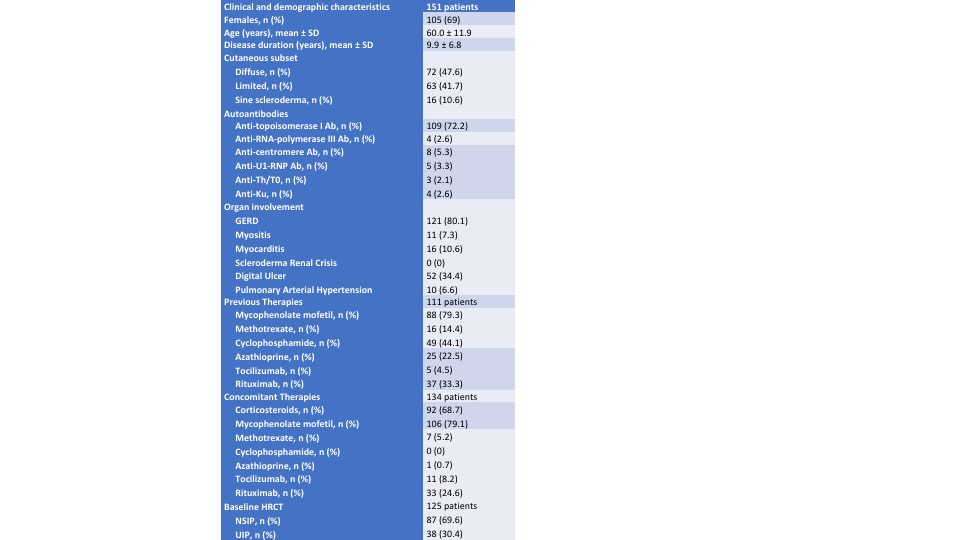
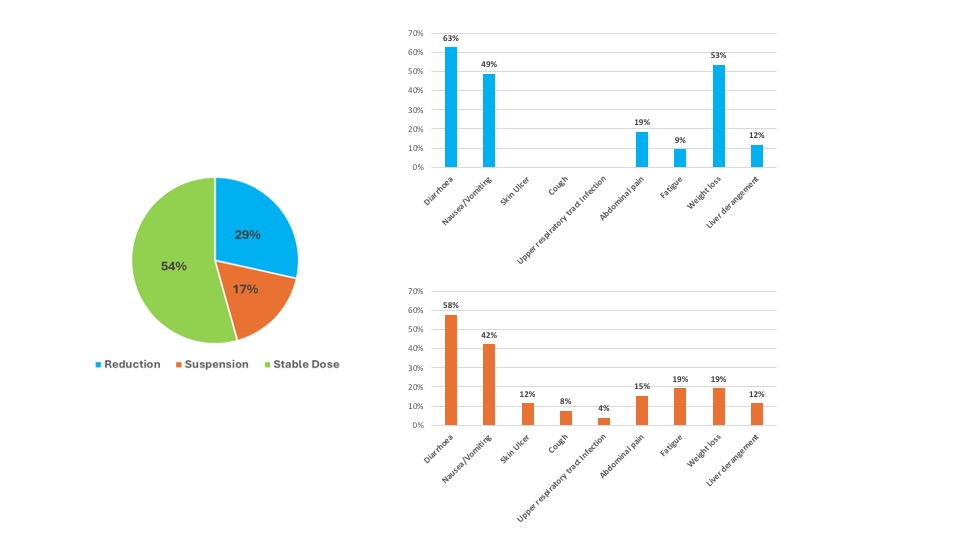
Results: 151 SSc-ILD patients treated with NTD were identified (see Table 1). In the 12 months prior to NTD treatment, a progressive phenotype was observed in 87/129 (67%) patients: in those who achieved 1-year follow-up (84 (66%) patients), the percentage of 1-year progressors was significantly lower compared to the previous 12 months (67% versus 24%, p< 0.001). In diffuse cutaneous systemic sclerosis (dcSSc), no significant change was observed for the mRSS at 12 months (9.5 ± 6.7 versus 10.3 ± 8.0, p=0.347). After a median time of 4 (2 – 8) months, NTD dose was reduced to 200 mg daily in 56 (37%) patients and was stable in 43 (77%) patients. In 26 (17%) patients NTD was stopped after a median time of 3 (1-9) months. At multivariate logistic regression analysis among baseline features, the use of high-dose proton pump inhibitors (PPI) (OR 3.263 (1.023 – 10.410) and mycophenolate mofetil (MMF) (OR 0.269 (0.091 – 0.795)) were associated with 12-month progression, whereas the presence of gastro-oesophageal reflux disease (OR 3.894 (1.048 – 19.623)) and use of high-dose PPI (OR 2.243 (1.010 –6.573)) were associated with NTD reduction/suspension. A total of 37 patients reached 2-year follow-up. At 2 years, the percentage of progressors did not change compared to the first year (27%, p=0.685). The prevalence of NTD-related side-effects and the causes of reduction and withdrawal are summarized in Figure 1. During the follow-up, 13 patients died after a median time of 12 (10 – 30) months.
Conclusion: In a real-life clinical scenario, NTD combined with immunosuppressants reduced the rate of progressors and this effect was maintained at 2 years. Combination with mycophenolate mofetil is associated with a better respiratory outcome whereas baseline use of high-dose PPI is a risk factor for poor drug tolerance and a worse respiratory outcome.
To cite this abstract in AMA style:
Campochiaro C, De Luca G, Lazzaroni M, Armentaro G, Spinella A, Bellocchi C, Ruaro B, Stanziola A, Benfaremo D, De Lorenzis E, Moccaldi B, Bonomi F, Bianchessi L, Iannone F, Cacciapaglia F, Beretta L, Guiducci S, Codullo V, Bosello S, Cuomo G, Zanatta E, Del Papa N, Airò P, Confalonieri p, Moroncini G, Giuggioli D, Dagna L, Matucci-Cerinic M. Predictors of Response and Long-term Efficacy and Safety of Nintedanib in Systemic Sclerosis-Interstitial Lung Disease: Data from an Italian Multicentre Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/predictors-of-response-and-long-term-efficacy-and-safety-of-nintedanib-in-systemic-sclerosis-interstitial-lung-disease-data-from-an-italian-multicentre-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-of-response-and-long-term-efficacy-and-safety-of-nintedanib-in-systemic-sclerosis-interstitial-lung-disease-data-from-an-italian-multicentre-study/