Session Information
Date: Sunday, November 13, 2022
Title: Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster II
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Understanding factors that impact response to therapy may help improve disease management, clinical outcomes and patient quality of life. Nails are often overlooked in psoriasis, despite being commonly affected in hard-to-treat disease and being associated with patient discomfort, functional impairment, psychological stress, and psoriatic arthritis risk.1,2 This post hoc analysis of the Phase 3 clinical trial VOYAGE 2 data evaluated the impact of baseline (BL) characteristics and Psoriasis Area and Severity Index (PASI) response at Week (W)16 on nail psoriasis response with guselkumab, an anti-interleukin 23 monoclonal antibody, treatment and during withdrawal and describes nail outcomes based on prior biologic exposure.
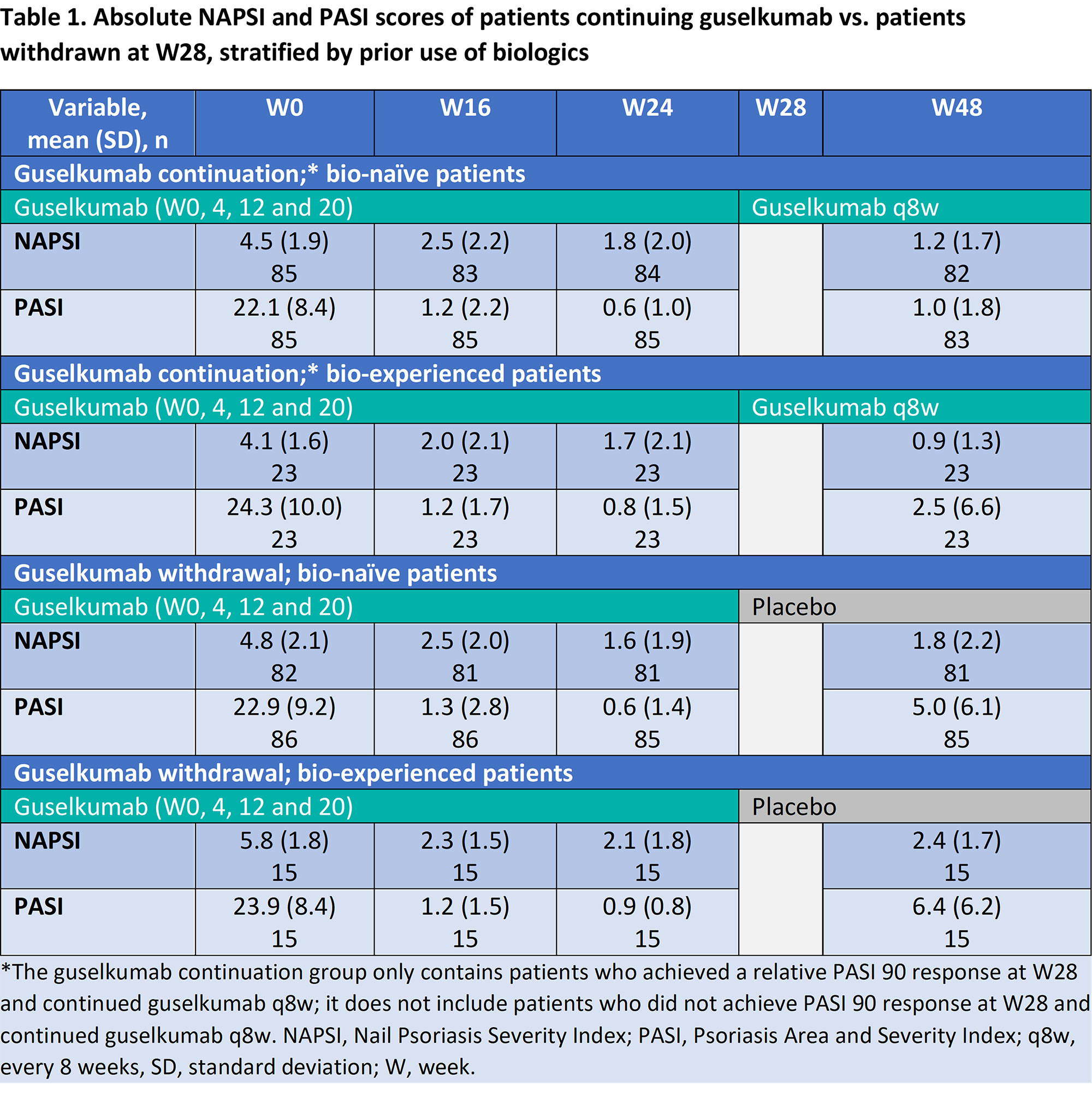
Methods: In VOYAGE 2, patients were randomised to guselkumab, placebo or adalimumab at BL; this analysis focussed on patients with fingernail psoriasis in the guselkumab arm. At W28, patients achieving PASI90 continued guselkumab every 8 weeks (q8w) or were withdrawn from treatment by receiving placebo; patients not achieving PASI90 continued guselkumab q8w. A logistic regression model established the association of BL characteristics and PASI response at W16 with attainment of Nail Psoriasis Severity Index (NAPSI)=0/1 at W24 and W48 for all patients receiving guselkumab. Additionally, for PASI90 responders at W28, absolute NAPSI and PASI up to W48 were reported for bio-naïve vs. bio-experienced patients. Comparative statistics of this post hoc analysis are nominal.
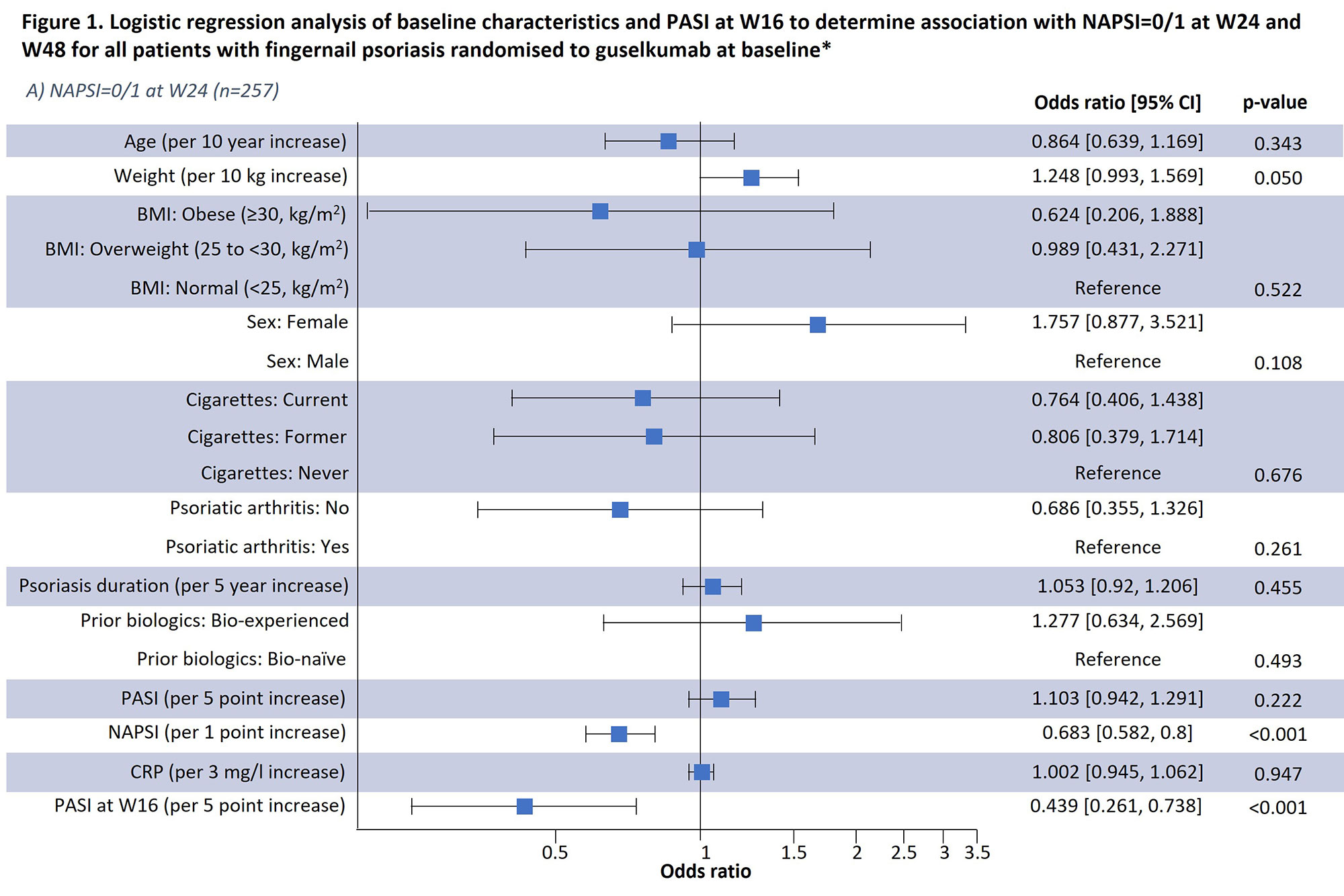
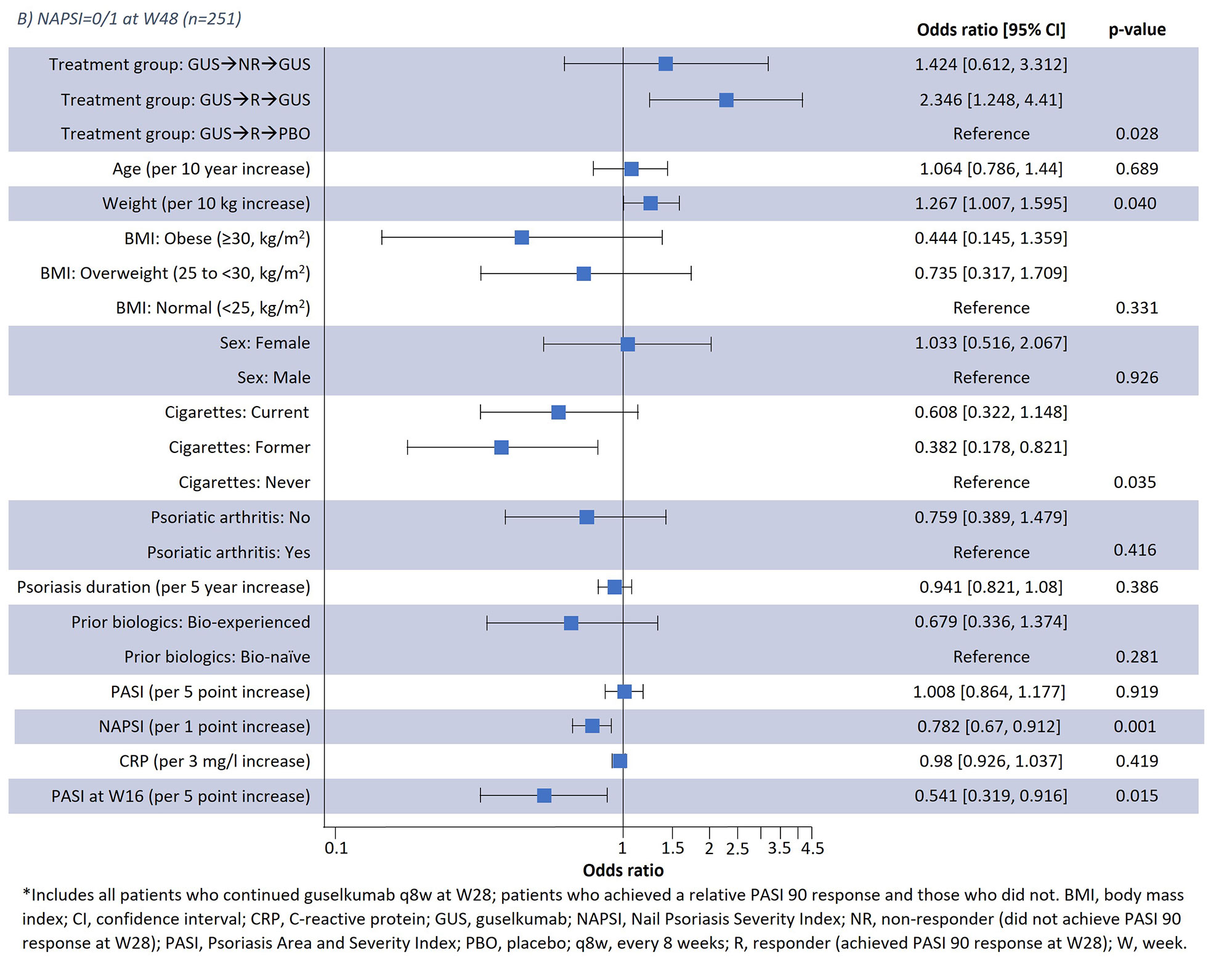
Results: Results of a logistic regression model of factors associated with NAPSI=0/1 at W24 and W48 in all patients receiving guselkumab (n=257) are shown in Fig1; lower BL NAPSI and W16 PASI were associated with achieving NAPSI=0/1 at W24 (p< 0.001 and p< 0.001, respectively) and W48 (p=0.001 and p=0.015, respectively). Moreover, smoking status was associated with NAPSI=0/1 at W48 (p=0.035); those with former and current smoking status had a lower probability of NAPSI=0/1 than those who had never smoked but this was not significant at W24. Increasing BL weight also showed a tendency towards greater probability of NAPSI=0/1 at W24 (p=0.05) and W48 (p=0.04). In a separate analysis of outcomes up to W48, previous biologic experience did not impact NAPSI response (Table 1); in patients continuing guselkumab, NAPSI continued improving between W24 and W48, whereas NAPSI remained stabled in the guselkumab withdrawal group.
Conclusion: Lower BL NAPSI and early skin improvements (lower W16 PASI) were associated with a higher probability of near or complete nail psoriasis clearance (NAPSI=0/1) at W24 or W48. Some association with NAPSI was observed for BL smoking status at W48. Association with weight requires further investigation due to the potential correlation with co-variables such as BMI. Being bio-experienced vs. bio-naïve was not associated with NAPSI=0/1 in the logistic regression analysis and did not impact NAPSI up to W48. These findings offer potential to inform clinical decisions in psoriasis disease management, such as assessing a patient’s probability of response to continuing therapy.
References
1. Egeberg A et al. BMC Dermatol 2020; 20: 3; 2. Baran R. Dermatology 2010; 221: S1–S5.
To cite this abstract in AMA style:
Egeberg A, Tillett W, Sonkoly E, Gorecki P, Tjärnlund A, Buyze J, Wegner S, McGonagle D. Predictors of Nail Response with Guselkumab: A Post Hoc Analysis of the VOYAGE 2 Clinical Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/predictors-of-nail-response-with-guselkumab-a-post-hoc-analysis-of-the-voyage-2-clinical-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-of-nail-response-with-guselkumab-a-post-hoc-analysis-of-the-voyage-2-clinical-trial/