Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: APS ACTION “Registry” was created to study the natural course of disease over 10 years in persistently antiphospholipid antibody (aPL)-positive patients with/without other systemic autoimmune diseases. Our objective was to determine the mortality rate as well as the causes and predictors of mortality in aPL-positive patients with/without APS classification.
Methods: A web-based data-capturing system is used to store patient demographics, history, and medications. The inclusion criteria are positive aPL according to the Updated Sapporo Classification Criteria tested within one year prior to enrollment. Patients are followed every 12 ± 3m with clinical data and blood collection. In this prospective analysis, firstly we analyzed descriptively the causes of death (based on investigators’ reports) for patients reported as “deceased” during the follow-up. Secondly, we compared the clinical and laboratory characteristics of deceased versus non-deceased patients using the adjusted Cox proportional hazards model and calculated the survival probability by Kaplan-Meier Model based on different age groups.
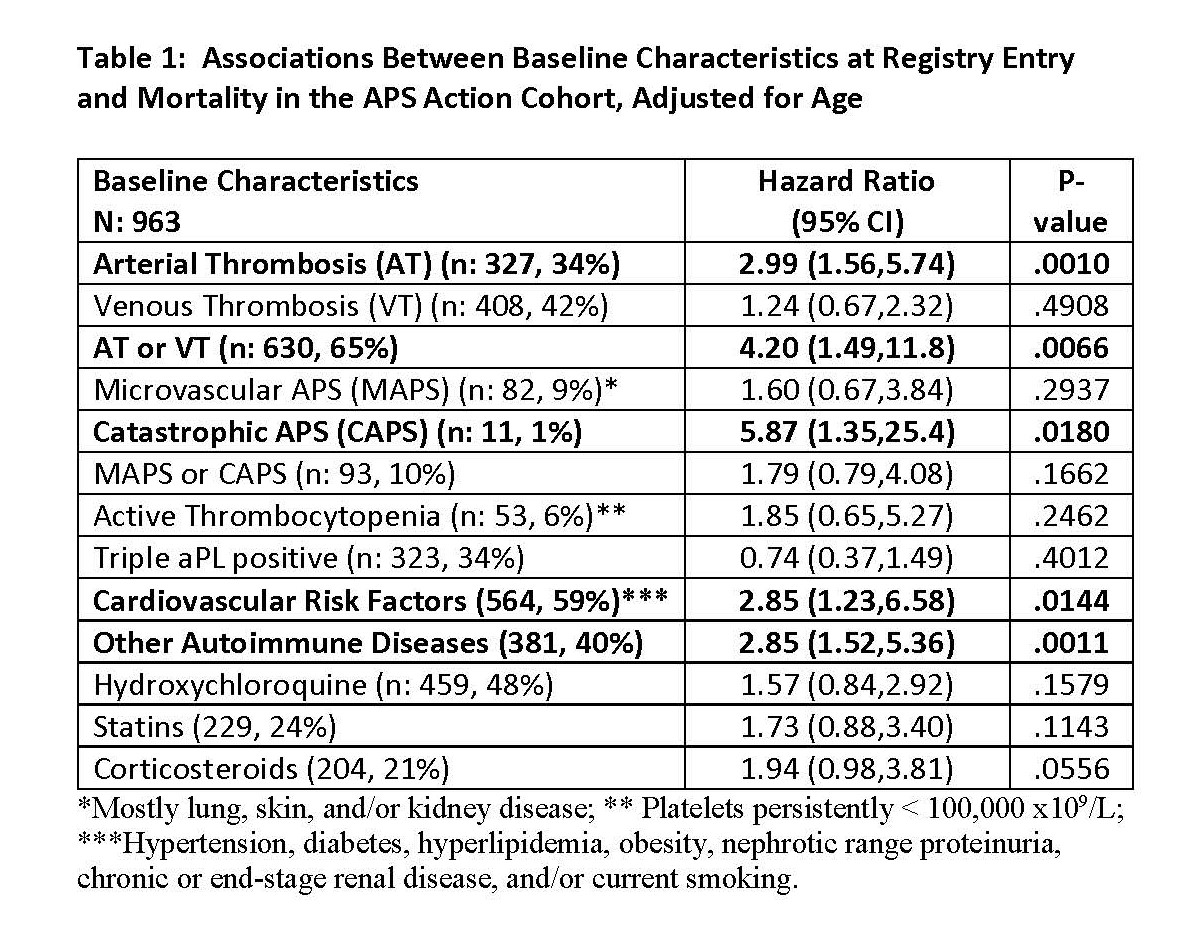
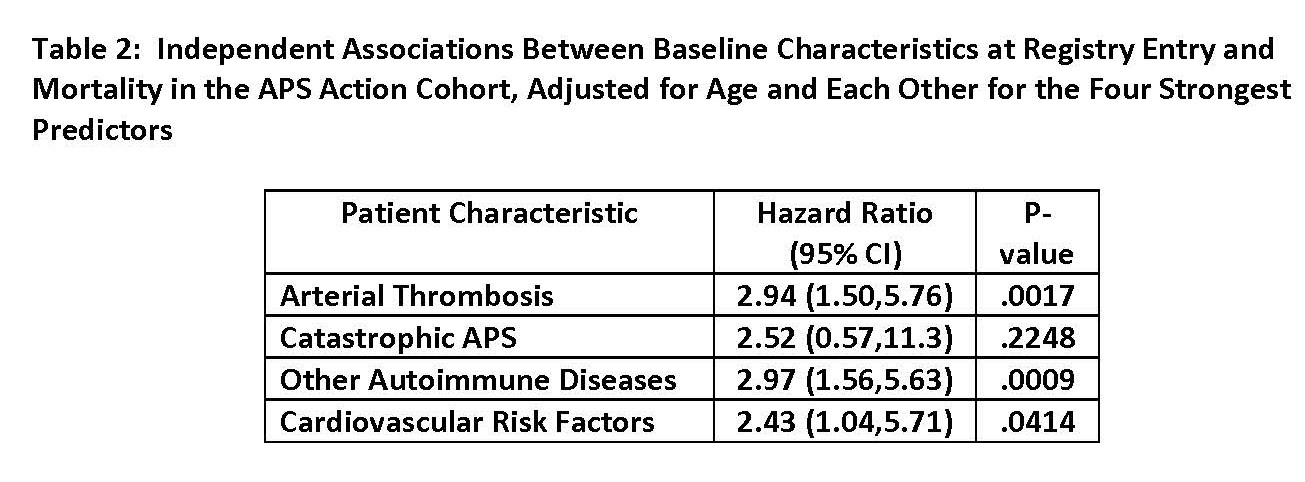
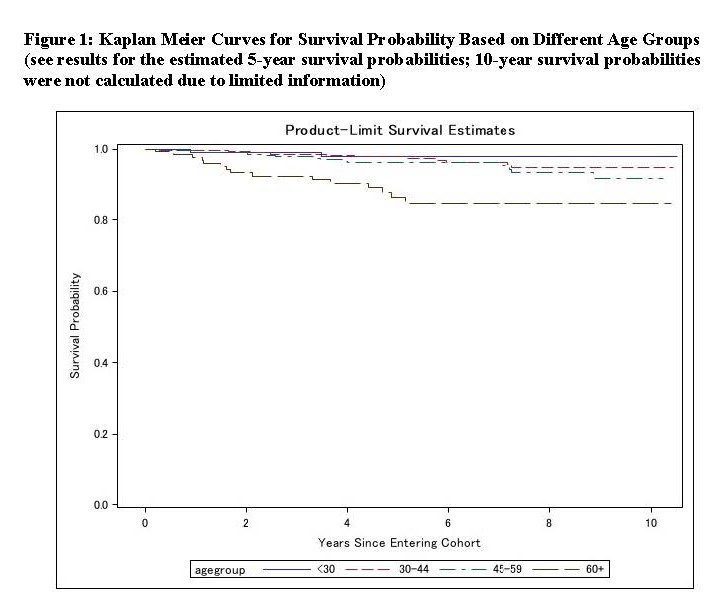
Results: As of May 2023, of 1,174 patients recruited, 215 (18%) were excluded due to incomplete follow-up data. Of the remaining 963 (mean age at registry entry: 53.0 ± 13.3, female: 723 [75%], and White: 657 [68%]), 43 (5%) were reported as deceased after a median follow-up of 5.3 years (interquartile range 2.4 to 7.9). The main causes of death (not mutually exclusive) were: infection (15, 35%), thrombosis (9, 21%), and malignancies (8, 19%). Based on the univariate analysis, history of arterial thrombosis or catastrophic APS (CAPS), selected baseline cardiovascular disease (CVD) risk factors, baseline active thrombocytopenia, and concomitant systemic autoimmune disease (SAID) were significantly more common in deceased patients, compared to non-deceased (data not shown). Based on the Cox proportional hazards model adjusted for age, arterial thrombosis, CAPS, CVD risk factors combined, and SAID were significantly and independently associated with mortality (Tables 1 and 2). Estimated 5-year survival probabilities starting from the registry entry (by age groups) were 0.98 (95% CI 0.92-0.99), 0.98 (0.95-0.99), 0.96 (0.93-0.98), and 0.86 (0.77-0.92) for ages < 30 (n:119), 30-44 (n: 362), 45-59 (n: 340), and >60 (n: 142), respectively (Figure 1).
Conclusion: Based on analysis of the largest multi-center international prospective cohort of persistently aPL-positive patients, the mortality rate was 5% after a median follow-up of five years. The estimated 5-year survival probability decreased with age; lowest (0.86) for patients over 60 years old at registry entry. History of arterial thrombosis, catastrophic APS, CVD risk factors, and concomitant systemic autoimmune diseases independently predicted future mortality
.
To cite this abstract in AMA style:
Ahmadzadeh Y, Magder L, Ozdemir Z, Andrade D, Barber M, Tektonidou M, Sciascia S, Pengo V, Anunciación-Llunell A, Ruiz-Irastorza G, Aguirre M, Belmont M, Nina K, Fortin P, WAHL D, Gerosa M, De Jesús G, Zhang Z, Atsuma T, Efthymiou M, Branch D, Tincani A, Rodriguez almaraz E, Pazzola G, Cervera R, Artim Esen B, Shi H, Knight J, Pons-Estel G, Willis R, Duarte-Garcia A, Bertolaccini M, Cohen H, Petri M, Erkan D, Of APS ACTION O. Predictors of Mortality in Antiphospholipid Antibody Positive Patients: Prospective Results from Antiphospholipid Syndrome Alliance for Clinical Trials and International Networking (APS ACTION) Clinical Database and Repository “Registry” [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/predictors-of-mortality-in-antiphospholipid-antibody-positive-patients-prospective-results-from-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-clinic/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-of-mortality-in-antiphospholipid-antibody-positive-patients-prospective-results-from-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-clinic/