Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Glucocorticoids (GCs) are a cornerstone of remission induction therapy in microscopic polyangiitis (MPA) and granulomatosis with polyangiitis (GPA), but long-term GC use is associated with serious adverse effects. Therefore, minimizing GC exposure while maintaining disease control is a major therapeutic goal. However, the frequency and predictors of glucocorticoid-free clinical remission (GFCR) within the first year after treatment initiation remains unclear in patients with newly diagnosed MPA/GPA. This study aimed to investigate the rate and predictors of GFCR at week 48 in patients with newly diagnosed MPA or GPA, and to examine long-term outcomes up to week 96.
Methods: We conducted a retrospective cohort study using a multicenter, nationwide registry in Japan (J-CANVAS). Patients with newly diagnosed MPA or GPA and at least 48 weeks of follow-up were included. GFCR was defined as Birmingham Vasculitis Activity Score (BVAS) of 0 with complete GC withdrawal at week 48. Univariable and multivariable logistic regression analyses were used to identify factors associated with GFCR. Given the limited prior evidence regarding predictors of GFCR, variables for multivariable models were selected based on clinical relevance and statistical trends in univariable analysis (Model 1: p < 0.05, Model 2: p < 0.20).
Results: Among 728 patients with newly diagnosed MPA/GPA enrolled in the registry, 544 were included in the analysis. At week 48, 29 patients (5.3%) achieved GFCR. Baseline characteristics are shown in Figure 1, and treatment details and outcomes up to week 48 are summarized in Figure 2. In multivariable analysis, the use of rituximab (RTX) and avacopan within the first 24 weeks was independently associated with achieving GFCR, whereas methylprednisolone pulse therapy was negatively associated (RTX: odds ratio [OR] 5.16, 95% confidence interval [CI] 2.37–11.22; avacopan: OR 14.67, 95% CI 4.81–44.70; methylprednisolone pulse: OR 0.14, 95% CI 0.03–0.61). Baseline characteristics including age, sex, ANCA serotype, disease activity (BVAS), and organ involvement were not significantly associated (Figure 3). Patients who achieved GFCR at week 48 were more likely to maintain GFCR at week 96 (58.6% vs. 4.5%, p < 0.001). Between weeks 48 and 96, the rates of death, relapse, and serious infection were similar regardless of GFCR status at week 48.
Conclusion: In this real-world cohort, the use of RTX and avacopan was independently associated with achieving GFCR at week 48, supporting their role in GC-sparing therapeutic strategies for AAV. Prospective studies are needed to validate these findings and guide individualized treatment approaches.
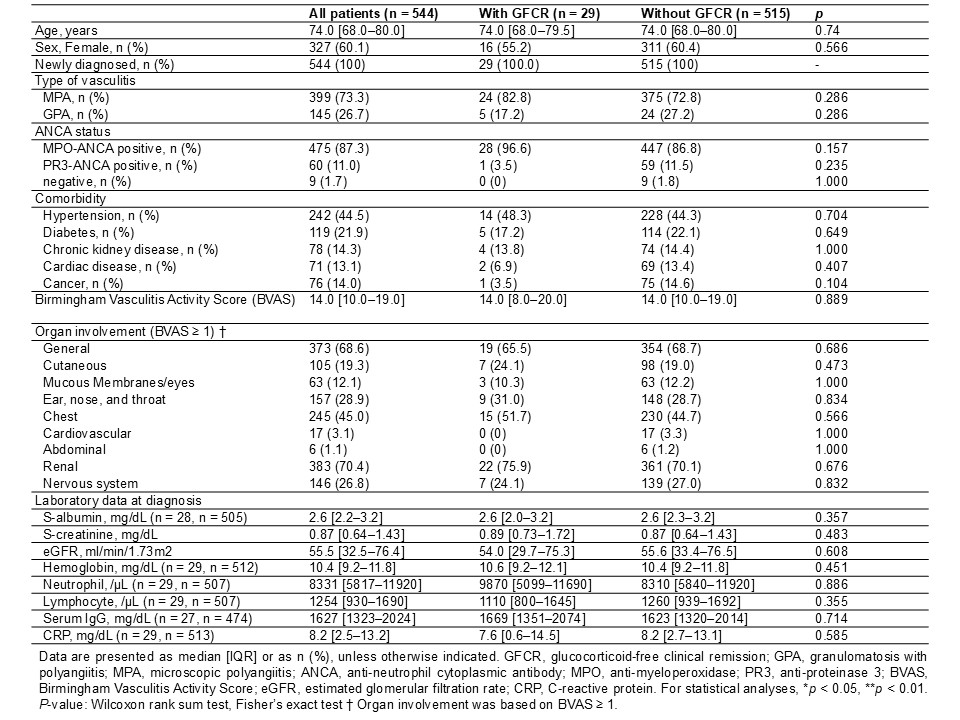 Figure 1. Comparison of baseline characteristics between patients with and without GFCR at week 48
Figure 1. Comparison of baseline characteristics between patients with and without GFCR at week 48
.jpg) Figure 2. Comparison of treatment details and outcomes up to week 48 between patients with and without GFCR at week 48
Figure 2. Comparison of treatment details and outcomes up to week 48 between patients with and without GFCR at week 48
.jpg) Figure 3. Univariable and multivariable logistic regression analyses for predictors of GFCR at week 48
Figure 3. Univariable and multivariable logistic regression analyses for predictors of GFCR at week 48
To cite this abstract in AMA style:
Ushio Y, Omura S, Nakagomi D, Abe Y, Wada M, Takizawa N, Nomura A, Kukida Y, Kondo N, Takagi H, Endo K, Hirata S, Azuma N, Takeuchi T, Fukui S, Kamada K, Yanai R, Matsuo Y, Shimojima Y, Nishioka R, Okazaki R, Takata T, Moriyama M, Takatani A, Miyawaki Y, Shirai T, Ito T, Matsumoto I, Takada T, Ito-Ihara T, Kida T, Yajima N, Kawaguchi T, Kawahito Y, Dobashi H. Predictors of Glucocorticoid-Free Clinical Remission at Week 48 in Newly Diagnosed Microscopic Polyangiitis and Granulomatosis with Polyangiitis: from nation-wide registry in Japan (J-CANVAS) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/predictors-of-glucocorticoid-free-clinical-remission-at-week-48-in-newly-diagnosed-microscopic-polyangiitis-and-granulomatosis-with-polyangiitis-from-nation-wide-registry-in-japan-j-canvas/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-of-glucocorticoid-free-clinical-remission-at-week-48-in-newly-diagnosed-microscopic-polyangiitis-and-granulomatosis-with-polyangiitis-from-nation-wide-registry-in-japan-j-canvas/
