Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: This post hoc analysis of the INVIGORATE-1 study evaluated potential predictors of Ankylosing Spondylitis international Society (ASAS) 40 response in patients with axial spondyloarthritis (axSpA) receiving intravenous (IV) secukinumab.
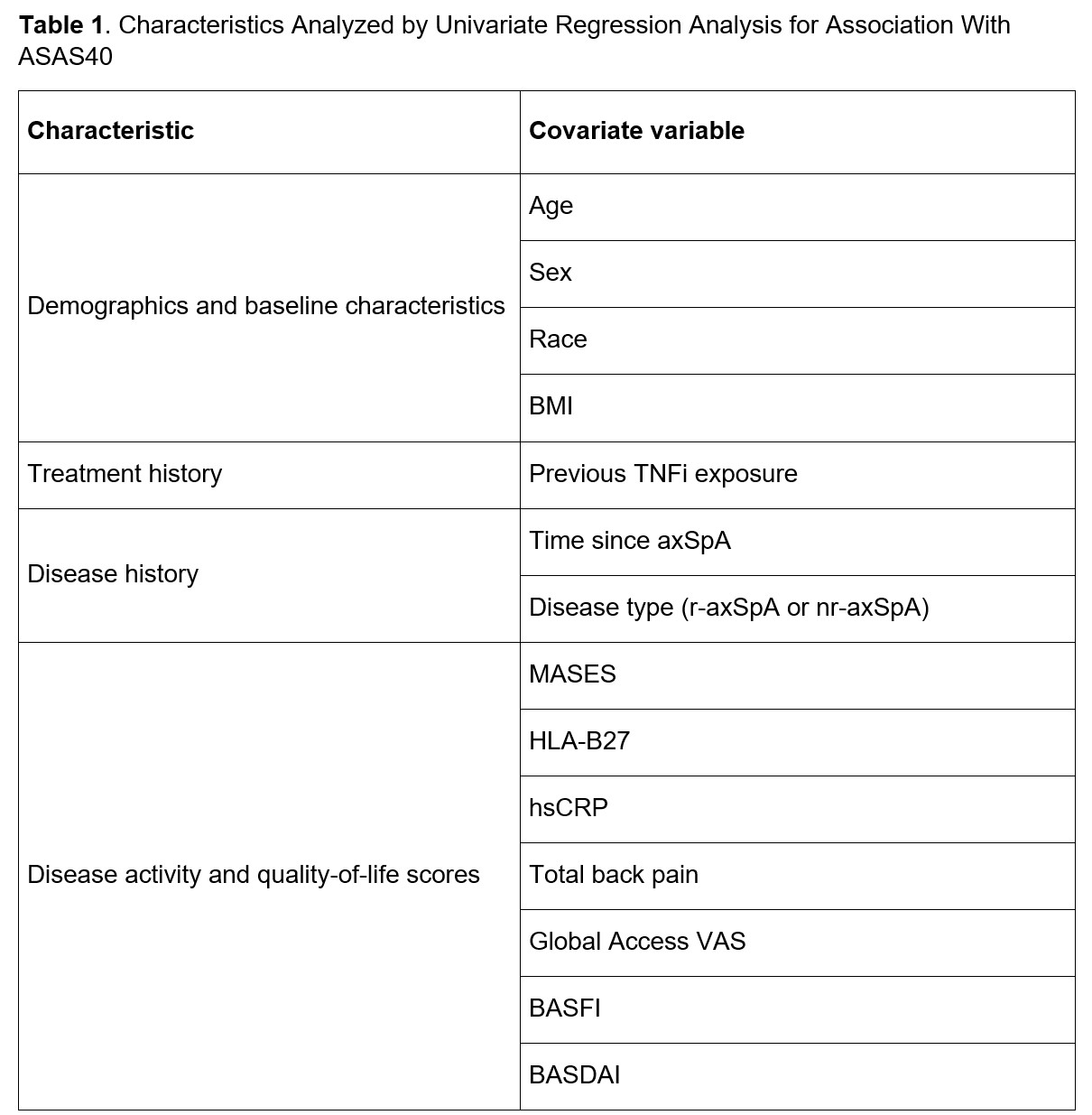
Methods: INVIGORATE-1 (NCT04156620) is a randomized, double-blind, parallel-group, phase 3 trial of patients with active axSpA (either radiographic [r] or non-radiographic [nr] axSpA). Enrolled patients were aged ≥18 years with a documented diagnosis of axSpA and fulfilled the ASAS classification criteria for axSpA (non-radiographic axSpA) or the 1984 Modified New York criteria for ankylosing spondylitis (radiographic axSpA). Patients were randomized 1:1 to IV secukinumab (6 mg/kg at baseline followed by 3 mg/kg every 4 weeks) or placebo for 16 weeks. Only patients randomized to IV secukinumab were included in this analysis. Univariate logistic regression analyses were performed to identify baseline characteristics (including demographics, disease characteristics, and treatment history; Table 1) that were likely associated (P< .2) with the achievement of ASAS40 through Week 16. These potential predictors were subsequently entered into a repeated measure multivariable logistic regression (RMMLR) model for further selection (P< .1 to keep). For each of the predictors kept by the RMMLR, descriptive subgroup summary of ASAS40 response rates through Week 16 are provided. All P values derived from this post hoc analysis are nominal.
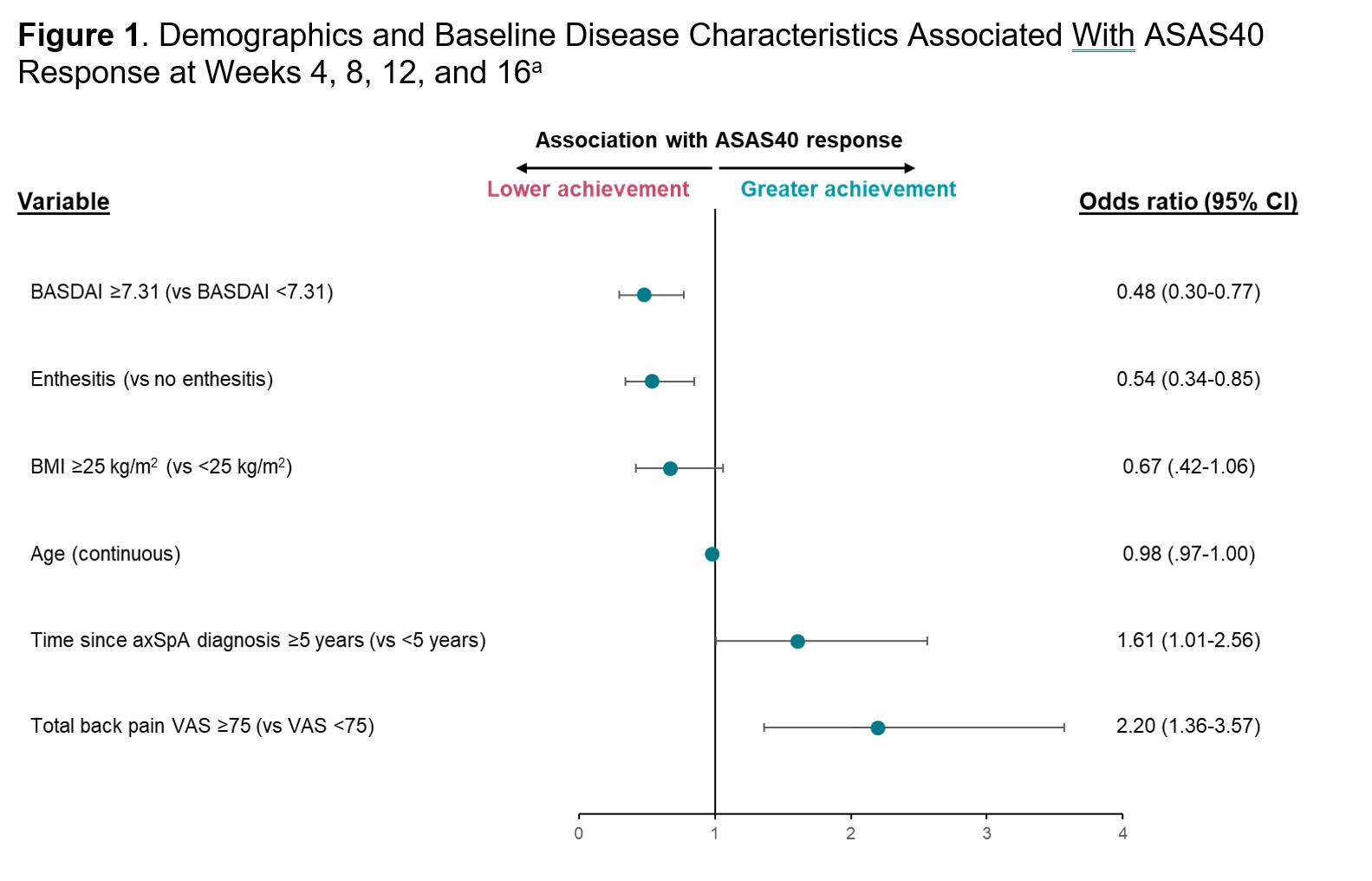
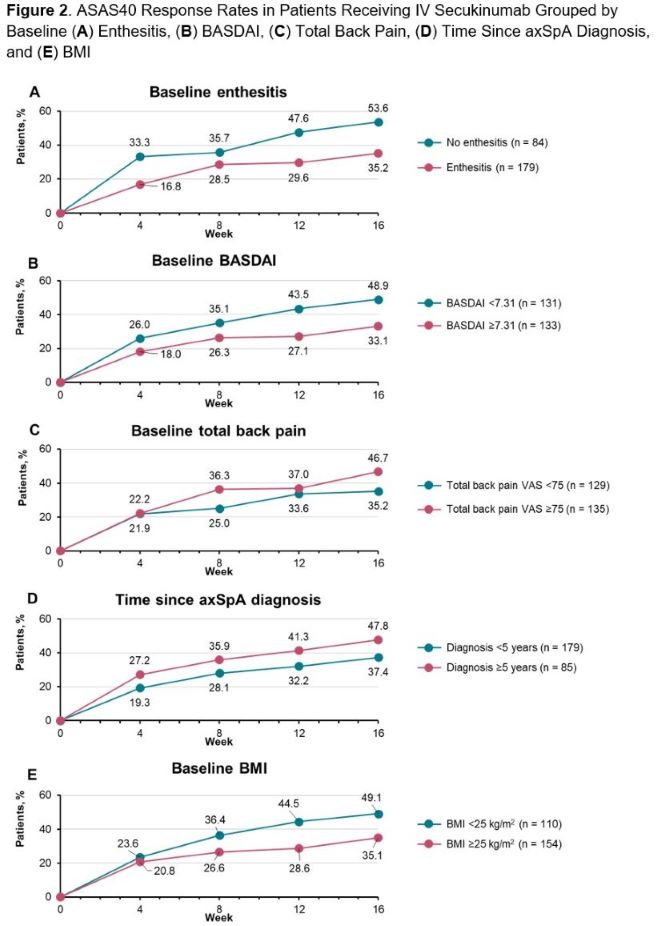
Results: This post hoc analysis included 264 patients with axSpA who received IV secukinumab in INVIGORATE-1. Greater baseline total back pain (visual analog scale [VAS] score ≥75 mm; odds ratio [95% CI]; 2.20 [1.36-3.57]; P< .01) and longer time since axSpA diagnosis (≥5 years; 1.61 [1.01-2.56]; P=.05) were associated with greater achievement of ASAS40 over 16 weeks of treatment, while baseline enthesitis (0.54 [0.34-0.85]; P< .01), greater baseline disease activity (BASDAI ≥7.31; 0.48 [0.30-0.77]; P< .01), and older age (continuous in years; 0.98 [0.97-1.00]; P=.05) were associated with lower achievement of ASAS40 (Figure 1). ASAS40 response rates from Weeks 4 to 16 were greater in patients without baseline enthesitis (vs those with enthesitis) and in patients with BASDAI < 7.31 (vs BASDAI ≥7.31), total back pain VAS ≥ 75mm (vs total back pain VAS < 75 mm), BMI < 25 kg/m2 (vs BMI ≥25 kg/m2), and time since diagnosis ≥5 years (vs time since diagnosis < 5 years) (Figure 2). Sex, HLA-B27 positivity, baseline hsCRP, previous TNF inhibitor exposure, and r-axSpA vs nr-axSpA status were not shown to be predictive of ASAS40 response.
Conclusion: This post hoc analysis demonstrated that higher levels of back pain, longer time since diagnosis, and lower BMI were associated with greater clinical response to IV secukinumab from Weeks 4 to 16 for patients with axSpA in INVIGORATE-1, while baseline enthesitis, older age, and greater disease activity were associated with lower achievement of clinical response. However, randomized clinical trial results demonstrated that patients treated with IV secukinumab had clinical benefit regardless of demographics or baseline disease characteristics.
ASAS, Assessment in Spondyloarthritis international Society; axSpA; axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Score; BMI, body mass index; MASES, Maastricht Ankylosing Spondylitis Enthesitis Score; VAS, visual analog scale.
a Selected from a repeated measure multivariate logistic regression model with ASAS40 at Weeks 4, 8, 12, and 16 as the response variable (P<.1).
Disclosures: A. Deodhar: AbbVie, 2, 5, 6, BMS, 2, 5, 6, Eli Lilly, 2, 5, 6, Janssen, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, UCB Pharma, 2, 5, 6; E. Dokoupilova: AbbVie/Abbott, 5, Eli Lilly, 5, Galapagos NV, 5, Gilead, 5, GlaxoSmithKlein(GSK), 5, Hexal AG, 5, Janssen-Cilag, 5, Nimbus, 5, Novartis, 5, Pfizer, 5, Sanofi, 5, UCB Biopharma SPRL, 5; C. Vizcaya: Novartis, 3, 8; R. Sutariya: Novartis, 3, 8; W. Bao: Novartis, 3, 11; K. Kapur: Novartis, 3, 8; S. Rohrer: Novartis, 3, 8; A. Kivitz: AbbVie, 2, 6, Amgen, 6, 11, Coval, 2, Ecor1, 2, Fresenius Kabi, 2, Genzyme, 12, Scientific ExpertGenzyme, Gilead, 2, 11, GlaxoSmithKlein (GSK), 2, 6, 11, Grunenthal, 2, Horizon, 2, Innovaderm, 2, Janssen, 2, Lilly, 6, Novartis, 11, Pfizer, 6, 11, Prime, 12, Educational, Prometheus, 2, Sanofi - Regeneron, 6, SynAct, 2, Takeda, 2, UCB, 2, 6, XBiotech, 2.
To cite this abstract in AMA style:
Deodhar A, Dokoupilova E, Vizcaya C, Sutariya R, Bao W, Kapur K, Rohrer S, Kivitz A. Predictors of Clinical Response to Intravenous Secukinumab Among Patients with Axial Spondyloarthritis: A Post Hoc Analysis of a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/predictors-of-clinical-response-to-intravenous-secukinumab-among-patients-with-axial-spondyloarthritis-a-post-hoc-analysis-of-a-phase-3-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-of-clinical-response-to-intravenous-secukinumab-among-patients-with-axial-spondyloarthritis-a-post-hoc-analysis-of-a-phase-3-trial/