Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
EULAR recommendations for RA therapy suggest addition of a biologic only if poor prognostic factors such as high disease activity are present. However, low baseline disease activity is associated with better biologic treatment outcomes. Better tools to predict remission/low disease activity (LDA) and aid in selection of patients for anti-TNF treatment are important.
Methods
GO-MORE was an open-label, multinational, prospective study in biologic-naïve patients (pts) with active RA despite DMARD therapy. Pts received 50-mg subcutaneous golimumab (GLM) once monthly for 6 months in addition to their background DMARDs. The following baseline characteristics were first evaluated in univariable models predicting 28-joint disease activity score based on ESR (DAS28-ESR) LDA and remission at 1 and 6 months: age, sex, smoking history, comorbidities, number of failed DMARDs, methotrexate dose, disease duration, tender joint count-28 (TJC28), swollen joint count-28 (SJC28), ESR, patient global assessment of disease activity (PGA), and HAQ. Factors were evaluated in a stepwise fashion. Those with significant associations (P<.10) were included in a final multivariable model. Factors that predicted both LDA and remission at 1 and 6 months were used in the final model. The ability of the models to predict LDA and remission at month 6 was investigated using receiver operating characteristic (ROC) analyses.
Results
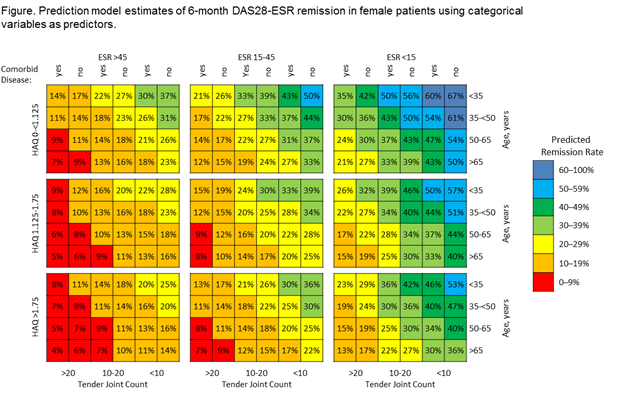
3280 pts were included in the analysis: 82.8% female, mean age 52.3 years, mean disease duration 7.6 years, mean baseline DAS28–ESR 5.97 (standard deviation=1.095). DAS28-ESR remission and LDA were achieved by 7.7% and 16.6% of pts, respectively, after 1 month of GLM treatment (1 injection), and by 23.9% and 37.4%, respectively, after 6 months of treatment. In multiple regression models, LDA at 1 month was associated with male sex; absence of comorbidities; and lower age, TJC28, SJC28, PGA, HAQ, and ESR. Remission at 6 months was associated with male sex; absence of comorbidities; and lower age, HAQ, ESR, and TJC28. The final model included sex, comorbidities, age, HAQ, ESR, and TJC28 as continuous variables and had an area under the ROC curve (AUC) of 0.81, 0.74, and 0.71 to predict remission at 1, 3, and 6 months, respectively, and an AUC of 0.80, 0.73, and 0.71 to predict LDA at 1, 3, and 6 months, respectively. When CRP replaced ESR or SJC replaced TJC, predictive ability was slightly reduced. The model for remission at 6 months had an AUC of 0.71 and showed remission rates from 4% to 67% in females (figure) and from 7% to 76% in males.
Conclusion
Sex, age, ESR, HAQ, absence of comorbidities, and TJC28 at baseline allow accurate prediction of remission and LDA in the first 6 months of GLM therapy in patients failing DMARDs. This prediction model allows better selection of anti-TNF candidates.
Disclosure:
N. Vastesaeger,
MSD Belgium,
3;
P. Durez,
None;
B. Dasgupta,
EULAR, ACR, Health Technology Assessment, British Heart Foundation, Research for Patient Benefits UK, and Napp,
2,
Schering Plough, Merck, Roche, Mundipharma, and Astra Zeneca,
5;
B. Combe,
Merck & Co., Inc.,
5;
H. Schulze-Koops,
Abbott, Actelion, Biotest, BMS, Chugai, Essex, GSK, MSD, Medac, Merck, Mundai Pharma, Novartis, Nycomed, Pfizer, Roche, and UCB,
5;
I. Louw,
Merck, BMS, Pfizer, Roche, Janssen, and Lilly,
2,
Abbott, BMS, Janssen Pharmaceutica in South Africa, and the Merck Investigator Consulting Network,
9;
J. Wollenhaupt,
MSD,
5,
MSD,
8,
AbbVie, UCB, Pfizer, Sanofi, and Astra Pharma,
2;
C. Zerbini,
Novartis, Pfizer, Bristol, Lilly, Amgen, and MSD,
5,
Pfizer, Bristol, Lilly, and MSD,
5,
Pfizer and Bristol,
6;
A. Beaulieu,
Merck, Servier, Novartis, Amgen, Abbott, Celgene, Pfizer, Eli Lilly, Roche Centocor, Novartis, AbbVie, UCB, and Arthrolab,
2;
K. Pavelka,
Amgen, Roche, BMS, MSD, and UCB,
9;
M. Lazaro,
Bristol Myers Squibb Argentina,
2,
Abbott Laboratories,
9,
MSD,
5;
A. Garcia Kutzbach,
Merck, Janssen, Lilly, Sanofi, and Astra Zeneca,
2;
R. Moots,
None;
H. Amital,
None;
S. Huyck,
Merck & Co., Inc.,
3;
B. Fu,
Merck & Co., Inc.,
3;
M. Govoni,
MSD Italy,
3;
H. Weng,
Merck & Co., Inc.,
3.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prediction-of-remission-and-low-disease-activity-in-dmard-refractory-patients-with-ra-treated-with-golimumab/