Session Information
Session Type: Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: A simple, scalable tool that identifies psoriasis patients at high risk for developing PsA could improve early detection and facilitate early intervention. Our overall objective is to develop an accurate risk prediction model for the development of PsA and to assess its performance among patients with psoriasis.
Methods: In this longitudinal cohort study we analyzed data from the International Psoriasis and Arthritis Team (IPART) study, a prospective cohort of psoriasis patients without PsA at the time of enrollment. The participants were followed prospectively from 2006 to 2020, and their PsA status was assessed annually by a rheumatologist. Information about their demographics, psoriasis characteristics, co-morbidities, medications and musculoskeletal symptoms was used to develop prediction models for PsA. Penalized binary regression models were used for variable selection while adjusting for psoriasis duration; the stacked LASSO with equal weights was adopted to deal with multiple imputed datasets for incomplete data. Risks of developing PsA over 1- and 5-year time horizons were estimated. Internal validity was assessed using 5-fold cross-validation. Model performance was assessed by the area under the curve (AUC), and calibration plots.
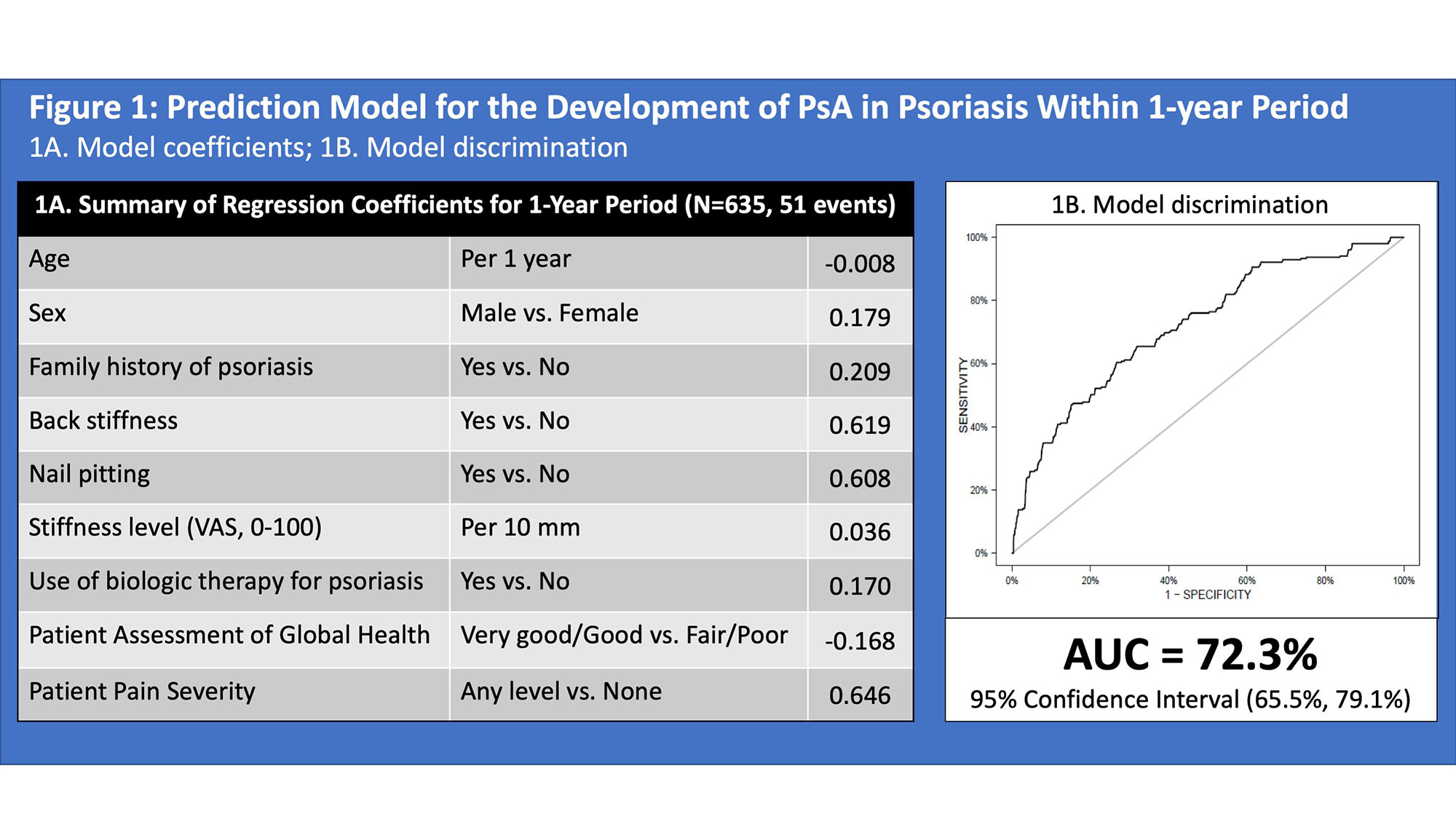
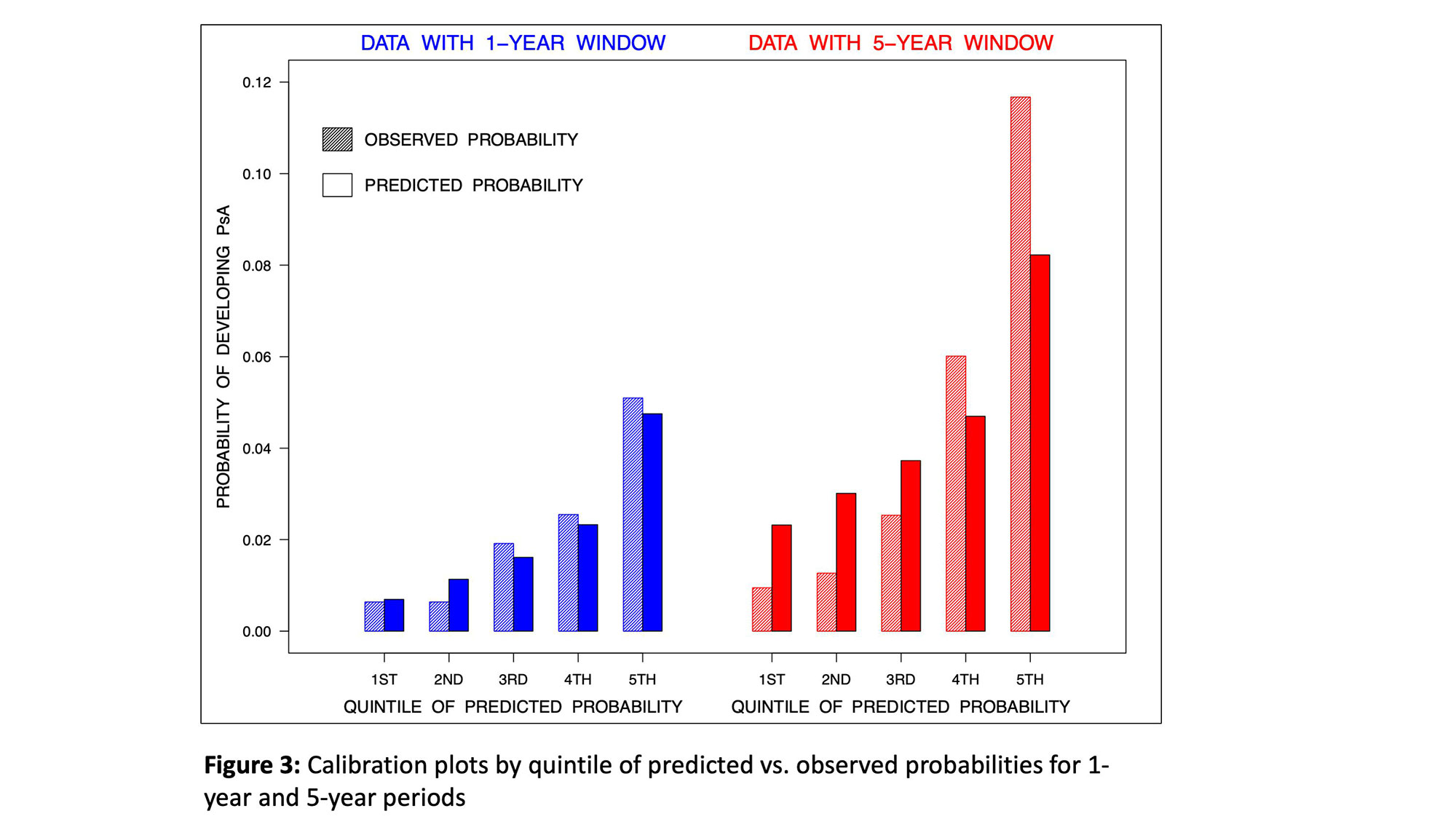
Results: A total of 635 psoriasis patients were analyzed (mean duration of follow up 7.7 years). 51 and 71 patients developed PsA during the 1-year and 5-year periods, respectively. The risk of developing PsA within 1 year was associated with younger age, male sex, family history of psoriasis, back stiffness, nail pitting, level of stiffness, use of biologic medications, global health and pain severity (AUC 72.3, 95% confidence interval ((CI) 65.5, 79.1, Figure 1). The risk of developing PsA within 5 years was associated with morning stiffness, psoriatic nail lesion, psoriasis severity (by PASI), fatigue severity (by FACIT-fatigue), pain severity and use of systemic non-biologic medication or phototherapy (AUC 74.9, 95% CI 69.3, 80.5, Figure 2). Calibration plots showed reasonable agreement between predicted and observed probabilities (Figure 3). The sensitivity and specificity for a 2.5% probability of PsA onset within 1 year were 54.5% and 75%, respectively. The sensitivity and specificity for a 5% probability of PsA onset within 5-years period were 61.1% and 77%, respectively.
Conclusion: The development of PsA within clinically meaningful time frames can be predicted with reasonable accuracy for psoriasis patients. Additional work is underway to validate these models in external cohorts of psoriasis patients.
To cite this abstract in AMA style:
Eder L, Lee K, Chandran V, Widdifield J, Drucker A, Ritchlin C, Rosen C, Cook R, Gladman D. Prediction of Psoriatic Arthritis Tool (PRESTO): Development and Performance of a New Scoring System for Psoriatic Arthritis Risk [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/prediction-of-psoriatic-arthritis-tool-presto-development-and-performance-of-a-new-scoring-system-for-psoriatic-arthritis-risk/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prediction-of-psoriatic-arthritis-tool-presto-development-and-performance-of-a-new-scoring-system-for-psoriatic-arthritis-risk/