Session Information
Date: Sunday, November 10, 2019
Title: RA – Diagnosis, Manifestations, & Outcomes Poster I: Risk Factors, Predictors, & Prognosis
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The multi-biomarker disease activity (MBDA) blood test has been shown to be a predictor of risk for radiographic progression in patients with rheumatoid arthritis (RA). Subsequently, the MBDA score was adjusted to account for the effects of age, sex and adiposity and was shown in two cohorts to be better than conventional disease activity measures for predicting risk for radiographic progression [Curtis, J.R., et al. Rheumatology (Oxford). 2018. PMID: 30590790]. Here, we combined data from four separate cohorts to validate the adjusted MBDA score as a predictor of radiographic progression. We also evaluated the ability of the adjusted MBDA score, as a continuous variable, to predict risk for radiographic progression.
Methods: Four cohorts with requisite data were identified and combined: the BRASS registry (N=401) and OPERA study (N=154), which have been previously evaluated; and the SWEFOT study (N=235) and Leiden registry (N=163), which are new to these analyses. The associations of radiographic progression (change per year in total Sharp score [ΔTSS]) with the adjusted MBDA score, seropositivity (RF and/or ACPA positive), DAS28-CRP, SDAI, CDAI, CRP, baseline total TSS, age, and sex, were evaluated using linear regression. Logistic regression was used to estimate risk for four levels of radiographic progression (ΔTSS >2, >3, >4 or >5), each as a function of the adjusted MBDA score.
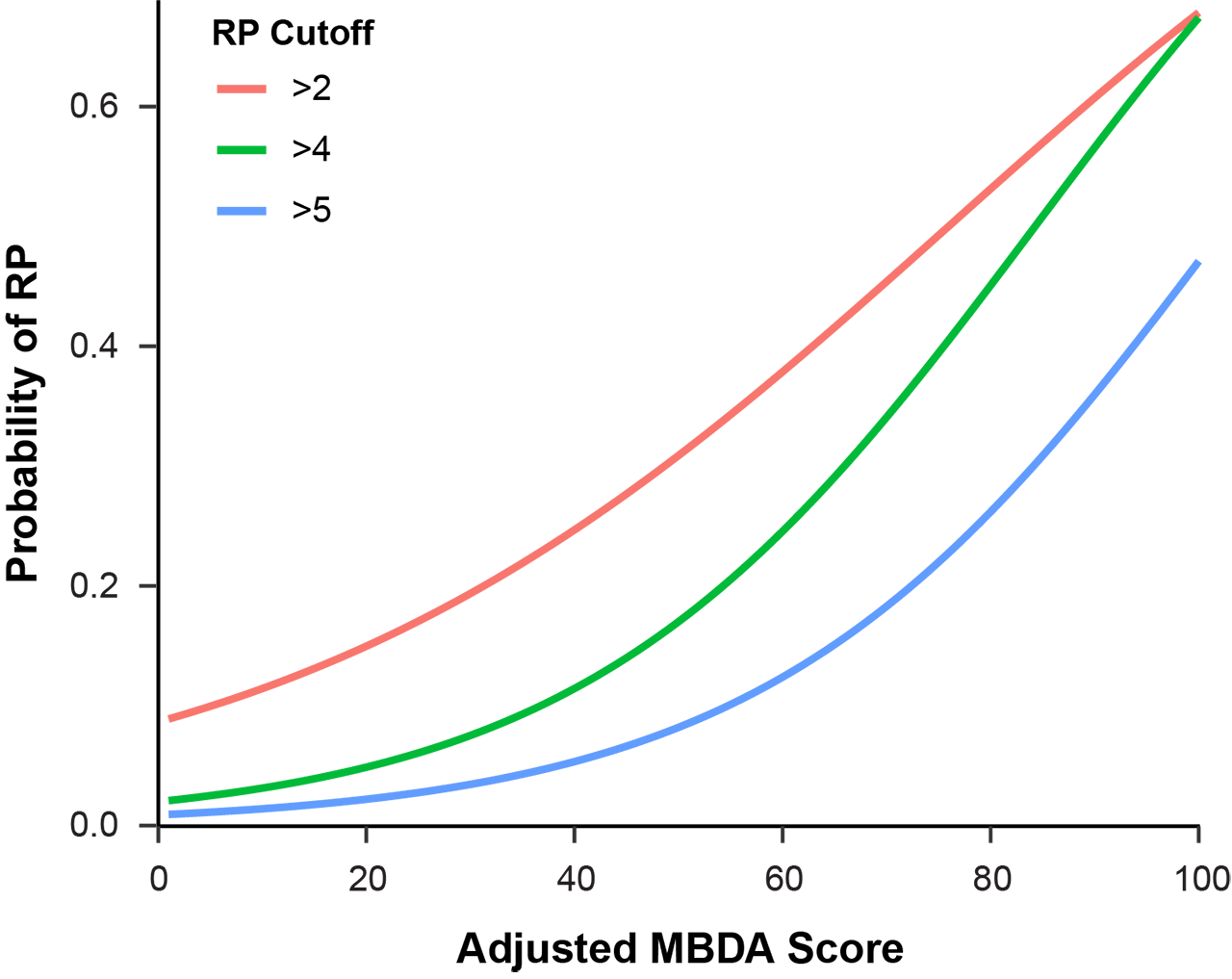
Results: The four cohorts were similar in age, adjusted MBDA score, and DAS28-CRP, with mean ages ranging from 55.2 to 56.4 years, mean adjusted MBDA scores from 39.9 to 42.5 and mean DAS28-CRP from 3.4 to 5.6. Patients in the OPERA and SWEFOT cohorts had early onset RA (mean durations 87 days and 6.1 months, respectively). Patients in the BRASS and Leiden cohorts tended to have established RA (mean durations 13.8 and 4.6 years, respectively). In a pooled analysis combining all four cohorts (N=953), the adjusted MBDA score was the most statistically significant univariate predicator of radiographic progression (p=2.5 x 10-13) among the tested measures of disease activity, followed by CRP (p=4.7×10-6) (Table 1). Curves were generated to display risk for four levels of radiographic progression, relative to the adjusted MBDA score as a continuous variable (Figure 1). The risk for the most severe level of progression that was evaluated, ΔTSS >5, ranged from 1% to 3% in the low (1-30) adjusted MBDA category to 7% to 47% in the high (45-100) adjusted MBDA category (Figure 1).
Conclusion: In a combined analysis of four cohorts of patients with RA, risk of radiographic progression (ΔTSS >5) was nearly absent when the adjusted MBDA score, as a continuous variable, was low, and exceeded 40% for patients with the highest MBDA scores.
To cite this abstract in AMA style:
Huizinga T, Weinblatt M, Shadick N, Brahe C, Østergaard M, Lund Hetland M, Hambardzumyan K, Saevarsdottir S, Horton M, Mabey B, Flake D, Gutin A, Ben-Shachar R, Sasso E, Curtis J. Predicting Risk of Radiographic Progression for Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/predicting-risk-of-radiographic-progression-for-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predicting-risk-of-radiographic-progression-for-patients-with-rheumatoid-arthritis/