Session Information
Date: Monday, October 22, 2018
Title: Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster II: Diagnosis and Prognosis
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
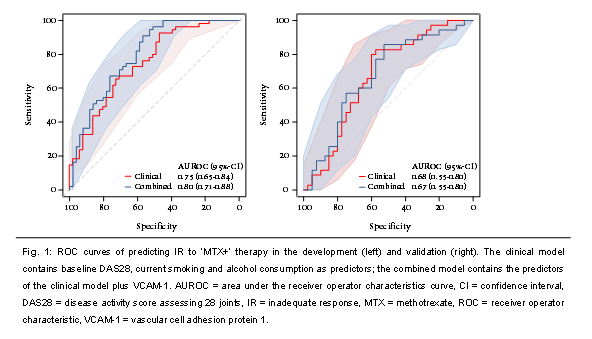
Results: None of the potentially relevant proteins (n=7) in the development sample were found to be significant (IR vs. no IR) in the validation sample. Although not statistically significant, the best performing biomarker was vascular cell adhesion protein 1 (VCAM-1, odds ratio (OR) 1.36, 95%-confidence interval (CI), 0.84-2.32; p=0.19), and was further investigated. In the development sample, the area under the receiver operator characteristics curve (AUROC) of the combined model (i.e. clinical predictors plus VCAM-1) was 0.80 (95%-CI 0.71-0.88): no significantly improved discriminative ability compared to clinical model (AUROC 0.75, 95%-CI 0.65-0.84; p=0.051). When applying the linear predictor to the validation sample, an AUROC of 0.67 (95%-CI 0.55-0.80) was found for the combined model: neither significantly different from clinical model (AUROC 0.68, 95%-CI 0.55-0.80). Conclusion: No protein biomarkers with additive value to clinical predictors for predicting IR to ‘MTX+’ in new-onset RA could be externally validated. The relatively easily obtainable clinical predictors still remain the most accurate factors to predict the need for adding a biologic DMARD to the initial MTX-based treatment strategy. References: 1. Teitsma XM, et al. Ann Rheum Dis 2018. May 14. [Epub ahead of print] 2. Teitsma XM, et al. Ann Rheum Dis 2016;75 (Suppl 2):440. 3. Bijlsma JWJ, et al. Lancet 2016 23;388(10042):343-355. 4. de Jong PH, et al. Ann Rheum Dis 2014;73(7):1331-1339.
To cite this abstract in AMA style:
Teitsma XM, Jacobs JWG, de Jong PH, Hazes J, Weel AE, Welsing PM, Pethö-Schramm A, Borm ME, van Laar J, Bijlsma JWJ, Lafeber FP. Predicting Response to Methotrexate Therapy in New-Onset RA: No Value of Adding Protein Biomarkers to Clinical Predictors [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/predicting-response-to-methotrexate-therapy-in-new-onset-ra-no-value-of-adding-protein-biomarkers-to-clinical-predictors/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predicting-response-to-methotrexate-therapy-in-new-onset-ra-no-value-of-adding-protein-biomarkers-to-clinical-predictors/