Session Information
Date: Sunday, November 10, 2019
Title: RA – Diagnosis, Manifestations, & Outcomes Poster I: Risk Factors, Predictors, & Prognosis
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) related autoantibodies, in particular antibodies to citrullinated proteins (ACPA), predict likelihood of developing future RA. Indeed, clinical trials for RA prevention are underway using ACPA positivity as an inclusion factor. However, there are multiple commercial ACPA assays including several versions of cyclic citrullinated protein (CCP) assays that may differ in diagnostic accuracy for future RA. For this project, we propose to use a novel sample set to evaluate the diagnostic accuracy in preclinical RA of 3 commercial CCP assays that are widely used in the USA.
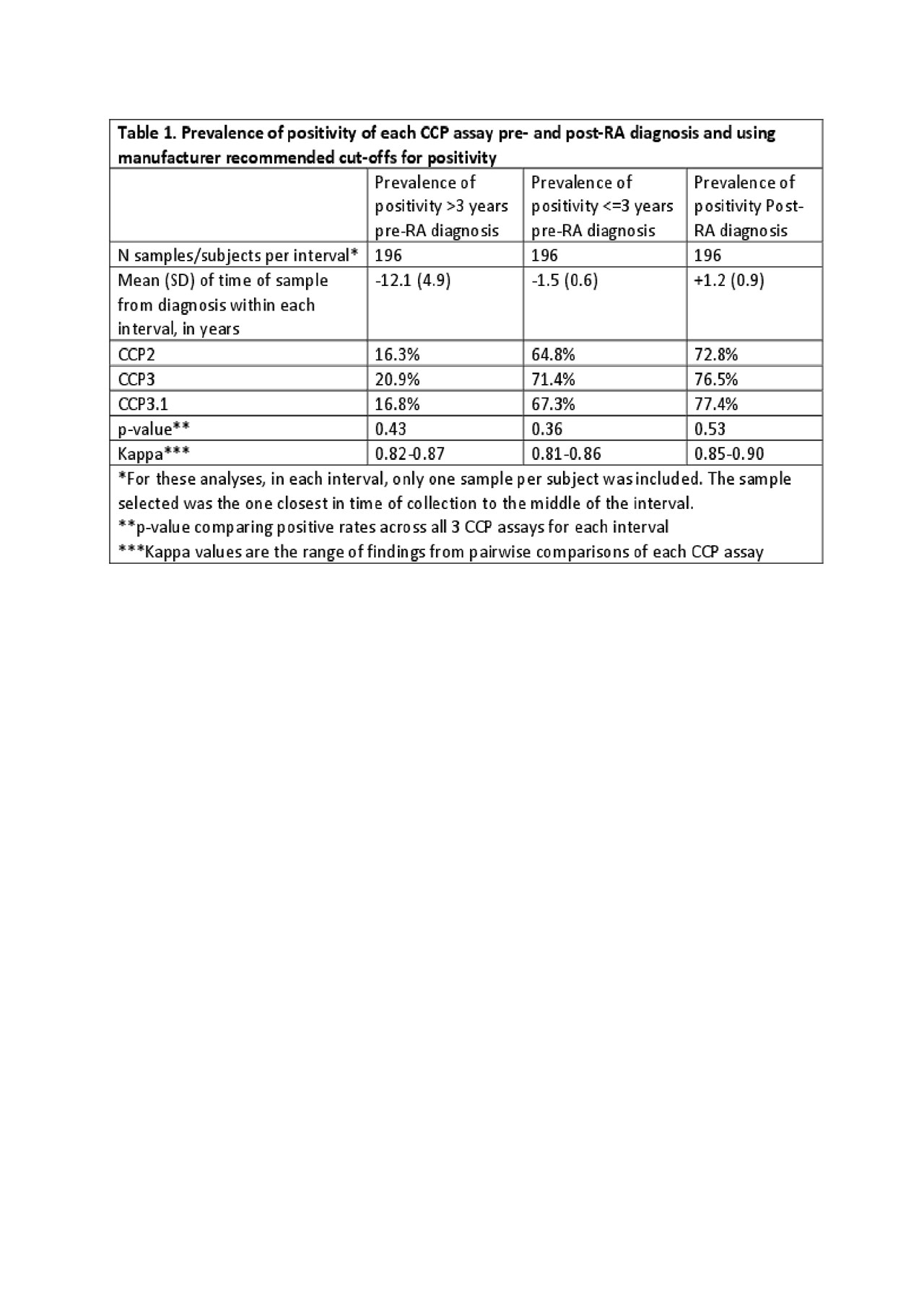
Methods: Cases who developed classifiable RA (n=196) during military service were identified and up to 3 pre-RA diagnosis and 1 post-RA diagnosis stored serum samples were selected from the Department of Defense Serum Repository along with serum from 208 matched controls. For the analyses herein, the pre-RA diagnosis Case samples were assigned as >3 or < =3 years from diagnosis, with each subject contributing only 1 sample per interval. Case and Control samples were tested using 3 commercially available CCP assays: CCP2 (ELISA IgG, Axis-Shield), CCP3 (ELISA IgG, Inova) and CCP3.1 (ELISA IgG/A, Inova) with positivity based on the manufacturer recommendations, and specificity determined in a single sample from Controls. The prevalence of CCP positivity in each interval was evaluated using three-way chi-square and kappa analyses. The relationship of positivity for a given test >3 and < =3 years of RA diagnosis was evaluated using a 2×2 table and Mantel-Haenszel odds ratio estimate.
Results: CCP2, CCP3 and CCP3.1 were positive in 72.8 to 77.4% of RA cases post-diagnosis (Table 1). The specificity, using Controls, of CCP2, CCP3 and CCP3.1 was 99.0, 98.6 and 96.6%, respectively; in addition, the specificity for all tests at >=2x the manufacturer recommended cut-off was 99%. In samples >3 years prior to diagnosis, the CCP assays were positive in 16.3 to 20.9% of Cases (Table 1). While CCP3 was positive in this interval in ~5% more subjects than CCP2 and ~4% than CCP3.1, these differences were not statistically significant. In samples < =3 years prior to diagnosis, the CCP assays were positive in 64.8 to 71.4% of Cases. While CCP3 was positive in this interval in ~7% more subjects than CCP2 and ~4% more subjects than CCP3.1, these differences were not statistically significant. For each CCP assay, cases were more likely to be positive < =3 years pre-RA diagnosis compared to >3 years (Table 2).
Conclusion: These results can help inform interpretation of CCP tests in individuals who may be positive in the absence of RA, as well as the choice of assay in clinical trials of RA prevention where the assay or cut-off level (e.g. >=2x) may impact diagnostic accuracy. Furthermore, the finding that positivity for these CCP assays was more likely to occur < =3 years prior to RA diagnosis is useful for informing CCP positive individuals about the potential timeline of development of RA, and in the development of clinical trials where this timeframe can inform trial design. Replication of these findings in additional cohorts is warranted, as well as comparison with additional ACPA assays that are in common use.
constitute endorsement or implied endorsement on the part of the author, DoD, or any component agency. The views expressed in this
abstract are those of the author and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or
U.S. Government.
To cite this abstract in AMA style:
Taylor S, Parish M, Moss L, Feser M, Mewshaw E, Edison J, Deane K. Pre-Rheumatoid Arthritis Diagnosis Prevalence of Commercial CCP Antibody Positivity Increases over Time with Strong Agreement Between Commercial Assays and Positivity Is Predicative of Developing Rheumatoid Arthritis Within 3 Years [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/pre-rheumatoid-arthritis-diagnosis-prevalence-of-commercial-ccp-antibody-positivity-increases-over-time-with-strong-agreement-between-commercial-assays-and-positivity-is-predicative-of-developing-rheu/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pre-rheumatoid-arthritis-diagnosis-prevalence-of-commercial-ccp-antibody-positivity-increases-over-time-with-strong-agreement-between-commercial-assays-and-positivity-is-predicative-of-developing-rheu/