Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Mycophenolate Mofetil is not recommended in pregnancy due to teratogenic effects. A previous small prospective study found that switching from MMF to AZA rarely led to renal flares. Our aim was to assess the impact of switching from MMF to AZA on renal flares and pregnancy outcomes in a lupus nephritis cohort.
Methods: A retrospective analysis of the records of patients attending the renal and antenatal clinics at St Thomas’s Hospital in London who underwent pre-pregnancy counselling and switched from MMF to AZA was undertaken between January 2019 and December 2020. Inclusion criteria included a diagnosis of SLE based on the 2019 EULAR/ACR classification criteria for SLE and lupus nephritis assessed by a biopsy and classified according to the International Society of Nephrology/Renal Pathology Society 2003 classification. A flare was defined as rising urine PCR, decreasing serum albumin, decreasing serum complement and rising dsDNA antibodies and/or an increase in serum creatinine. Analyses included descriptive statistics [mean (standard deviation) and median (interquartile ranges) for continuous variables and frequency (percent) for categorical values].
Results: Twenty-seven patients were included in this study. 55% were of Afro-Caribbean or Asian background and remaining were Caucasian. The median age at SLE diagnosis was 22 (18-26) years. The average disease duration prior to treatment switch was 8 years. The median time between biopsy and switching was 32 months (17-69). 25.9% of patients had a diagnosis of APS. 48% were Ro positive, while 85.2% were positive for dsDNA antibodies. The median serum creatinine was 69 umol/L (58-84), median serum albumin was 42g/L (38-47) and median PCR was 26.5mg/mol (12-61) prior to switching. Our study showed that 9 patients (33.3%) flared after switching (Table 1). 5 patients (18.5%) flared during pregnancy. 10 (37%) patients went onto have successful pregnancies, 2 (7.4%) had miscarriages, 3 (11.1%) patients developed preeclampsia. 46% of patients with class III/IV Nephritis on biopsy developed complications in pregnancy. 36% of patients with dsDNA had poor pregnancy outcomes compared to 25% who were dsDNA negative. 42.2% of patients of Afro-Caribbean/Asian background flared during pregnancy compared to 25% of their Caucasian counterparts.
Conclusion: Although Azathioprine is an option in patients with lupus nephritis who are planning pregnancy, there is a need to identify patients at risk of a disease flare on switching from MMF. This study showed that patients with low serum albumin, creatinine >65, urine PCR >50 and positive dsDNA antibodies at the time of the switch were more likely to flare after switching and in pregnancy.
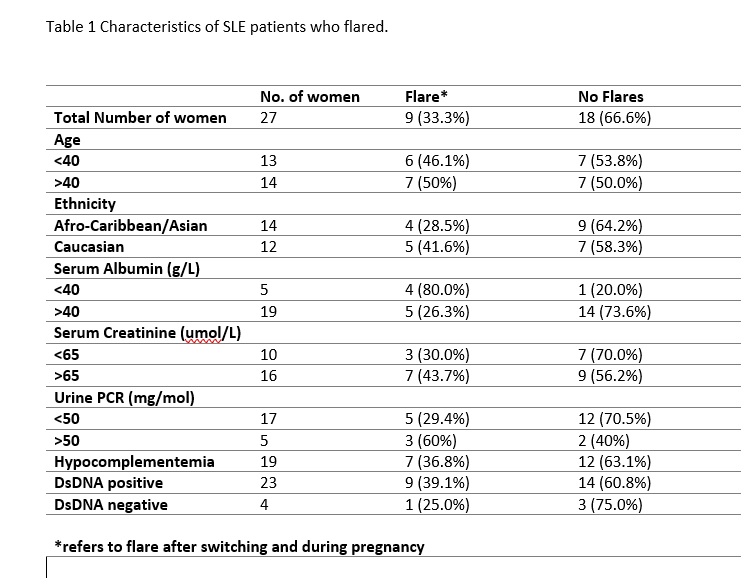 Table 1 Characteristics of SLE patients who flared.
Table 1 Characteristics of SLE patients who flared.
To cite this abstract in AMA style:
Ali S, Sangle S, Al Falah M, D'Cruz D. Pre-pregnancy Switching from Mycophenolate to Azathioprine in Patients with Lupus Nephritis: A Retrospective Outcome Analysis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/pre-pregnancy-switching-from-mycophenolate-to-azathioprine-in-patients-with-lupus-nephritis-a-retrospective-outcome-analysis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pre-pregnancy-switching-from-mycophenolate-to-azathioprine-in-patients-with-lupus-nephritis-a-retrospective-outcome-analysis/
