Session Information
Date: Monday, November 14, 2022
Title: Abstracts: Epidemiology and Public Health II: COVID, Infection and Pregnancy Outcomes
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Humoral responses to SARS-CoV-2 vaccination are reduced in patients with immune-mediated inflammatory diseases (IMID), especially when treated with B-cell depletion therapies, leading to increased likelihood of severe COVID-19 infection (1,2). A pre-exposure prophylaxis (PrEP) with monoclonal antibodies against SARS-CoV-2 has been shown to efficiently prevent symptomatic COVID-19 in the general population (3). Therefore, PrEP against COVID-19 has the potential to be used for the passive immunization of IMID patients who did not respond to SARS-CoV-2 vaccinations.
Purpose: To assess the persistence of anti-SARS-CoV-2 IgG antibodies in serum and saliva of IMID patients not responding to vaccination after receiving a PrEP with casirivimab/imdevimab. SARS-CoV-2 infection, adverse COVID-19 outcomes, and safety were investigated as secondary outcomes.
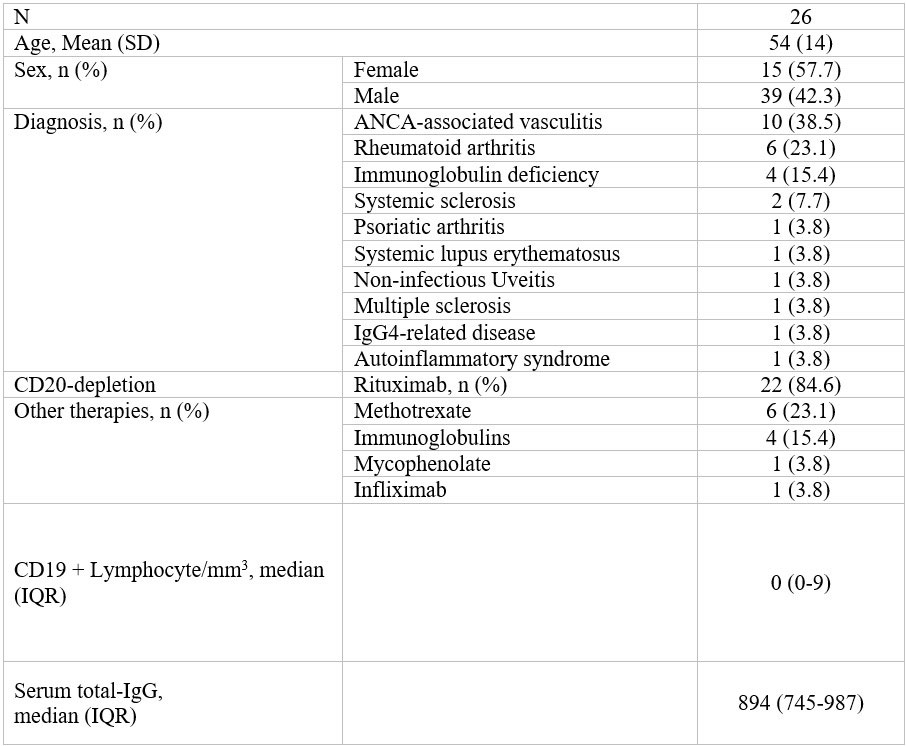
Methods: We studied anti-SARS-CoV-2 IgG titers in IMID patients treated with a PrEP of 1200 mg subcuateneous casirivimab/indevimab. All participants had failed to mount an adequate anti-SARS-CoV-2 humoral response at least 21 days after the third COVID-19 vaccination and were thus considered at high-risk of infection (Table 1). Anti-SARS-CoV-2 Spike IgG levels in serum and saliva were measured by ELISA (EUROIMMUN, Lübeck, Germany) before PrEP and at days 1, 14 and 30. IgG levels were calculated as antibody ratios by dividing the sample´s optical density by that of the calibrator. A positive threshold of > 1.1 was used. SARS-CoV-2 infection confirmed by polymerase chain reaction (PCR) and adverse COVID-19 outcomes such as hospitalization, mechanical ventilation and death as well as safety profile were reported.
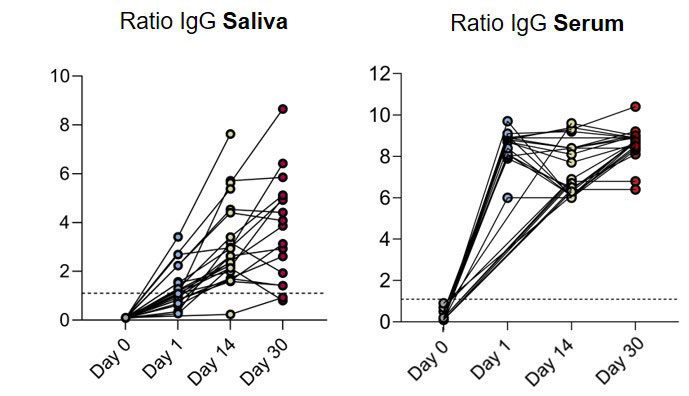
Results: We collected 92 serum and 75 saliva samples from 26 individuals at four consecutive timepoints (Figure 1). Anti-SARS-CoV-2 IgG titers were detected in all individuals´ serum and saliva samples, from day 1 and for the next 30 days after PrEP, regardless of diagnosis, therapy, total serum IgG or peripheral circulatingCD19+ B-cells. Serum IgG increased rapidly above the positivity threshold at day 1 and remained stable from day 14 to 30 (Figure 1, right panel), reaching levels comparable to those found in healthy individuals after full vaccination (1). Saliva IgG levels grew steadily from administration day to day 14 and then reached a plateau at day 30 (Figure 1, left panel). There were no side effects recorded. Five participants (19.2%) had a close contact with a SARS-CoV-2 infected person. All except for one remained asymptomatic and tested negative on PCR. The patient who tested positive experienced a mild COVID-19 with fever and cough.
Conclusion: SARS-CoV-2 PrEP leads to stable antibody levels in the serum and saliva of IMID patients who failed to respond to COVID-19 vaccination, independently of pre-existing clinical and serological characteristics. PrEP with casirivimab/imdevimab dipslays a safe clinical profile and has the potential to prevent infection and severe COVID-19 in high-risk patients refractory to vaccination.
SD, standard deviation; IQR, interquartile range; ANCA, anti-neutrophil cytoplasmic antibodies
To cite this abstract in AMA style:
Fagni F, Schmidt K, Bohr D, Valor Mendez L, Minopoulou I, Yalcin Mutlu M, Hartmann F, Tascilar K, Manger K, Manger B, Kleyer A, Simon D, Schett G, Harrer T. Pre-exposure Prophylaxis for SARS-CoV-2 with Subcutaneous Casirivimab/Imdevimab in Vaccine-refractory Patients with Immune-mediated Inflammatory Diseases [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/pre-exposure-prophylaxis-for-sars-cov-2-with-subcutaneous-casirivimab-imdevimab-in-vaccine-refractory-patients-with-immune-mediated-inflammatory-diseases/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pre-exposure-prophylaxis-for-sars-cov-2-with-subcutaneous-casirivimab-imdevimab-in-vaccine-refractory-patients-with-immune-mediated-inflammatory-diseases/