Session Information
Date: Monday, November 18, 2024
Title: T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic Lupus Erythematosus (SLE) is distinguished by immune system dysfunction, leading to heightened activation of T and B cells. This increased activity leads to the generation of auto-antibodies and the accumulation of immune complexes. Anomalous T lymphocyte activation, stemming from signaling irregularities, impaired gene transcription, and altered cytokine production, is a key player in the pathogenesis of SLE. Understanding these defects in signaling and gene regulation in SLE T cells can help identify new molecular targets and predictive biomarkers for therapy. PP2A is a critical serine/threonine phosphatase in eukaryotic cells, with distinct subunits known to influence T cell development and function, potentially contributing to autoimmune disorders. This research focuses on exploring the role of the regulatory subunit PPP2R3C in the aberrant function of SLE T cells.
Methods: Quantitative RT-PCR and western blotting were used to analyze changes in PPP2R3C mRNA and protein levels in SLE CD4+ T cells. Jurkat stable transfection cell lines were established with shRNA-PPP2R3C and overexpression-PPP2R3C through lentiviral transfection. Transcriptome sequencing was then conducted to identify differentially expressed genes associated with PPP2R3C. Flow cytometry was used to assess the impact of PPP2R3C on T cell activation, proliferation, and cellular calcium influx upon TCR stimulation. The TCR-mediated T cell activation pathways affected by PPP2R3C were further analyzed using western blotting. An overexpression model was generated using an adeno-associated virus carrying PPP2R3C, followed by pristane induction to evaluate the therapeutic potential of PPP2R3C in the SLE disease model.
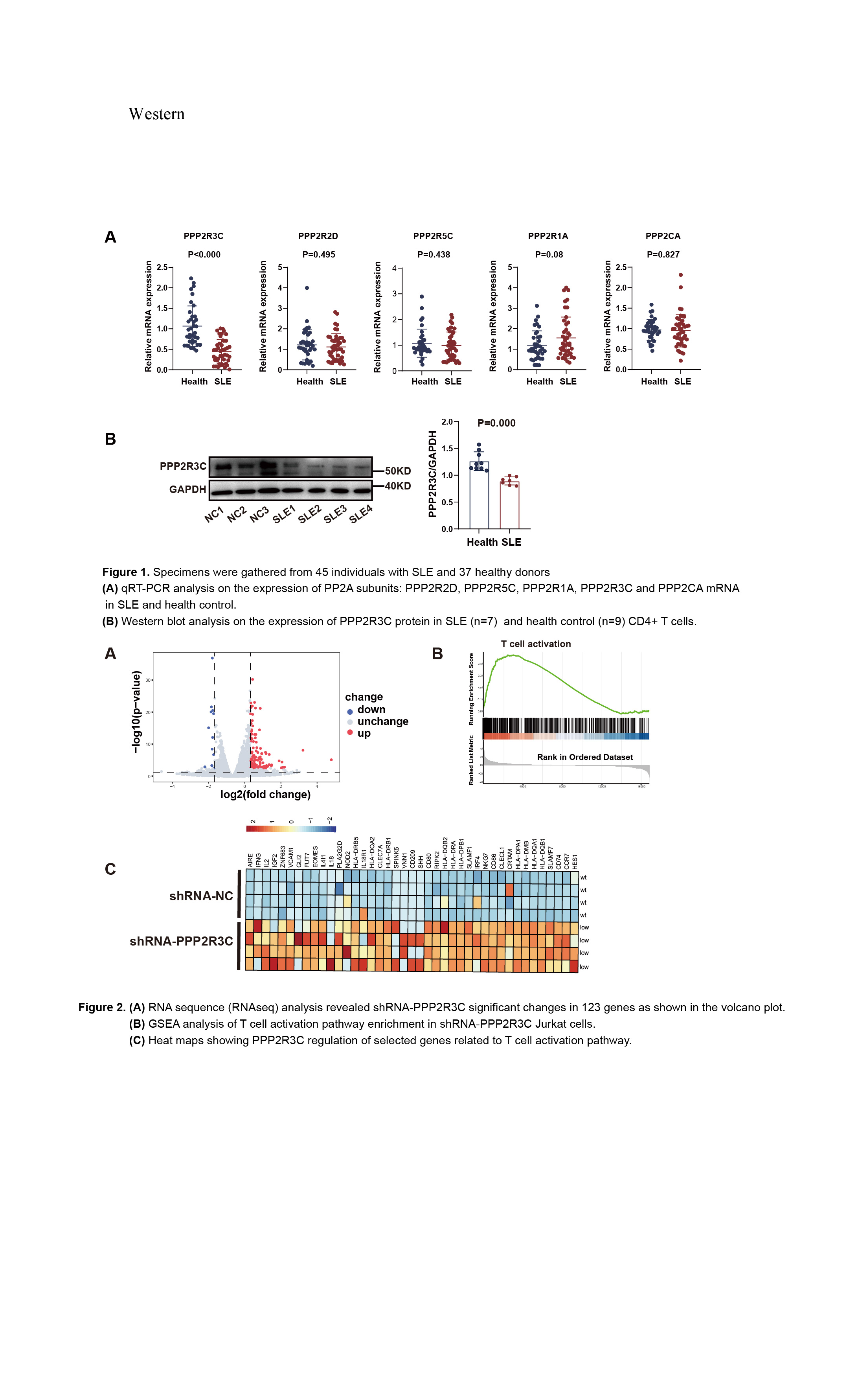
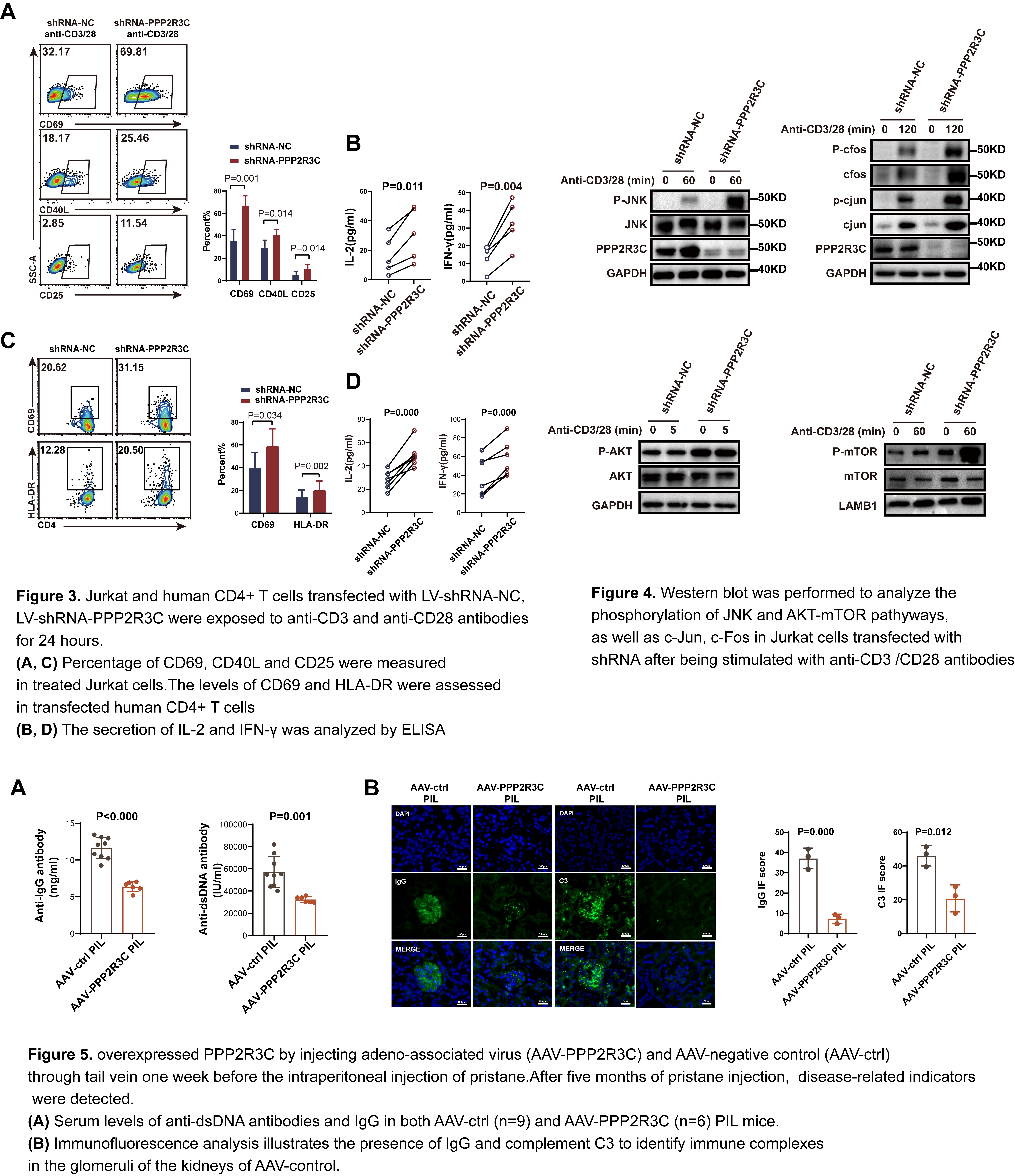
Results: Lower levels of PPP2R3C were observed in CD4+ T cells from SLE patients and PIL mice compared to non-autoimmune controls(Figure 1A,B). RNA-seq analysis showed upregulation of T cell activation–related pathway genes in shRNA-PPP2R3C Jurkat cells (Figure 2A-C). Both Jurkat cells and human CD4+ T cells displayed increased T-cell activation upon PPP2R3C removal, leading to elevated IL-2 and IFN-γ cytokine release upon TCR activation (Figure 3A-D). Mechanistic studies revealed that PPP2R3C depletion enhanced AKT-mTOR and JNK-cJun signaling pathways activation post-TCR stimulation (Figure 4). In vivo experiments with PPP2R3C overexpression in pristane-induced SLE (PIL) mice showed a reversal of disease biomarkers and improvement in lupus-like features(Figure 5A,B).
Conclusion: Our study indicates that the decrease in PPP2R3C expression may contribute to the pathogenesis of SLE by enhancing the activation of CD4+ T cells via TCR-triggered AKT-mTOR and JNK-cJun pathways. Modulating PPP2R3C levels could be a viable therapeutic strategy for controlling CD4+ T cell activation in SLE.
To cite this abstract in AMA style:
Fang X, Li X, Li X. PPP2R3C Overexpression Suppresses TCR -mediated CD4+ T Cell Abnormal Activation in Systemic Lupus Erythematosus via JNK and AKT-mTOR Pathways [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/ppp2r3c-overexpression-suppresses-tcr-mediated-cd4-t-cell-abnormal-activation-in-systemic-lupus-erythematosus-via-jnk-and-akt-mtor-pathways/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ppp2r3c-overexpression-suppresses-tcr-mediated-cd4-t-cell-abnormal-activation-in-systemic-lupus-erythematosus-via-jnk-and-akt-mtor-pathways/