Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
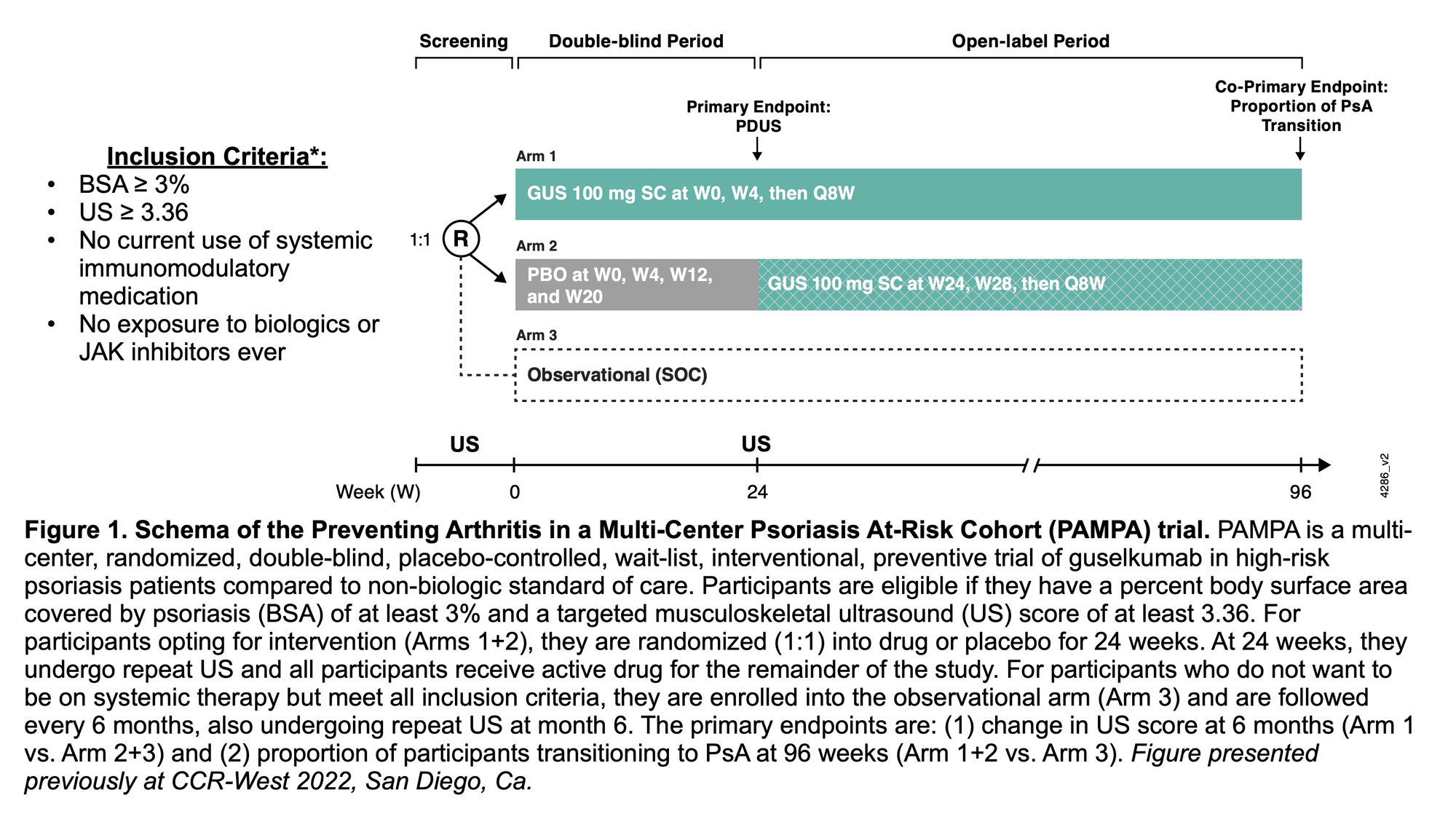
Background/Purpose: Psoriatic arthritis (PsA) is an immune-mediated disease associated with skin psoriasis that, if untreated, can lead to joint destruction. Up to 30% of patients with psoriasis progress to PsA and, in most cases, psoriasis precedes synovio-entheseal inflammation, providing a unique opportunity for early and potentially preventive intervention in a susceptible and readily identifiable population. The ongoing Preventing Arthritis in a Multicenter Psoriasis At Risk cohort (PAMPA) study (NCT05004727) aims to evaluate the efficacy of the fully human IL-23p19-subunit inhibitor guselkumab in preventing PsA and reducing musculoskeletal ultrasound (US) abnormalities in a population of patients with psoriasis at increased risk of PsA progression (Figure 1). Interim findings from screening US evaluations to date are reported.
Methods: Patients were screened across 5 sites in the US and Canada. PAMPA inclusion required ≥ 3% body surface area (BSA) affected by psoriasis, no current systemic immunosuppressant therapy at time of screening or prior exposure to biologic/JAK inhibitor, and evidence of musculoskeletal abnormalities on US. The US scanned a set of 36 joints and 34 periarticular structures (including tendons and entheses), each scored by 2 independent central readers using the Rochester Modified (RM)-PsASon scoring system (range 0-614). Participants required a mean score of ≥ 3.36 to enroll (the maximized Youden’s index based on previous analysis of scores in healthy controls and patients with psoriasis).
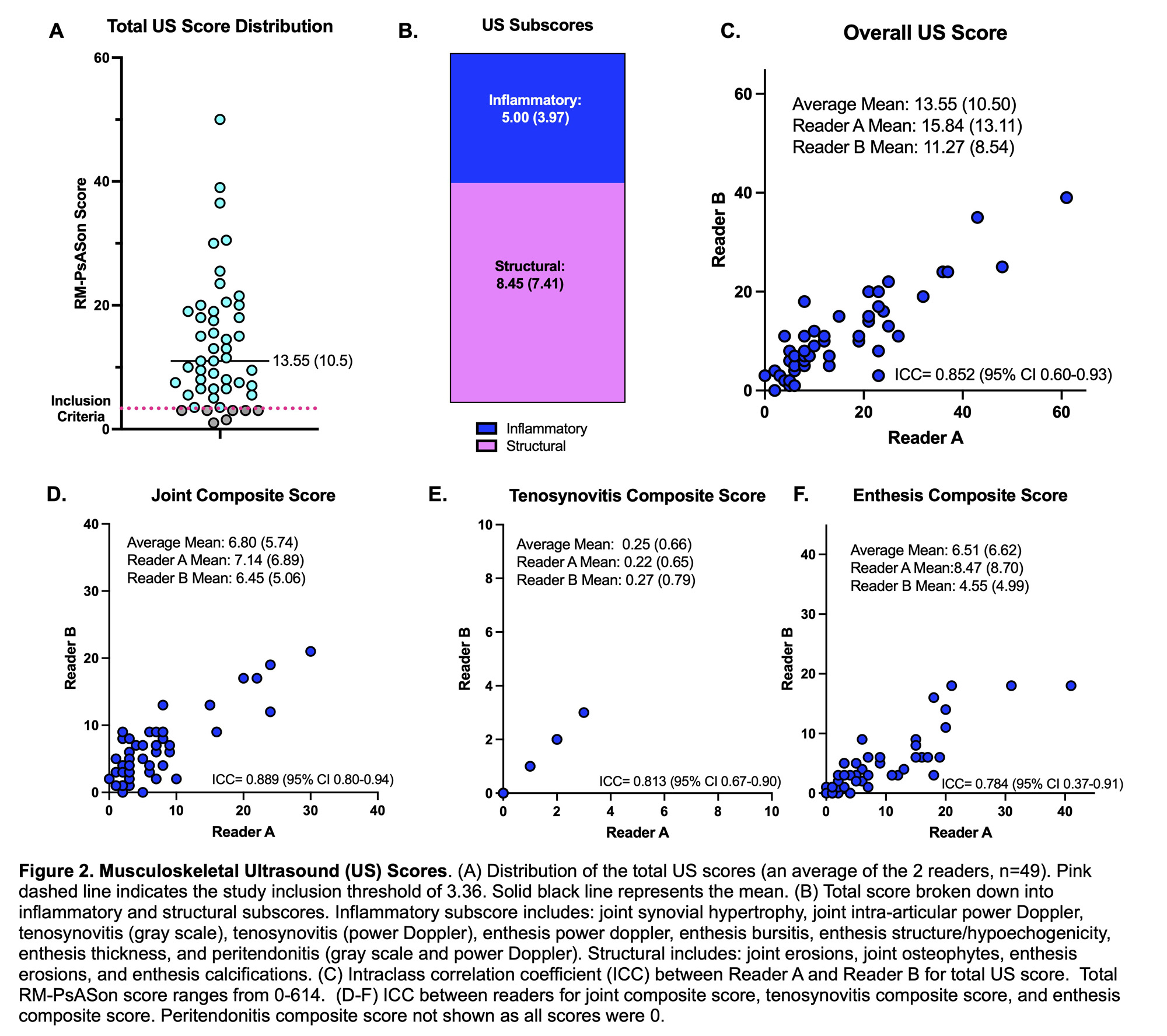
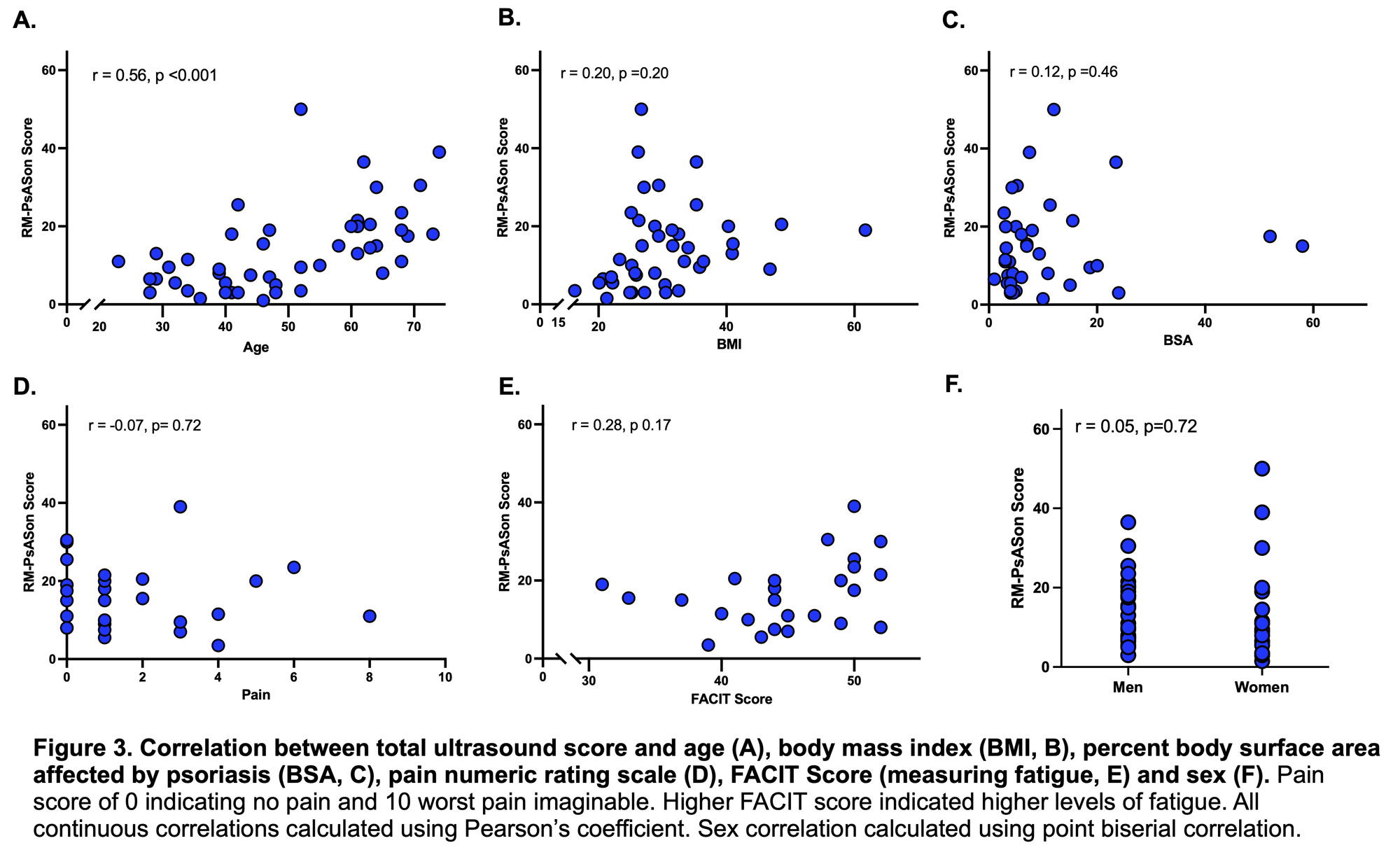
Results: Among the 49 participants screened to date, 42 met US criteria. The screened population was mostly male (57.1%), white (83.7%), with an average age of 49.2 (SD 14.3) and a body mass index (BMI) of 30.6 (SD 8.6). Participants had mostly plaque psoriasis (95.1%) at the time of enrollment with a mean BSA of 10.4% (SD 11.9). The mean total US score was 13.55 (SD 10.5, range 1-50), composed of inflammatory and structural subscores (Figure 2A-B). The intraclass correlation coefficient (ICC) assessing reader agreement was 0.89 (95% CI 0.80-0.94) and ICCs on joints, tendons, and entheses were all greater than 0.75 indicating excellent reliability (Figure 2C-F). US scores correlated with increasing age (r = 0.55, p= < 0.001), but did not correlate with BMI, BSA, FACIT-Fatigue score, patient reported pain intensity, or sex (Figure 3).
Conclusion: In a biologic-naïve cohort of patients with psoriasis at increased risk for PsA progression, 85.7% of those with US evaluation exceeded a score of 3.36, a previously identified threshold to distinguish those with psoriasis from healthy controls. The interrater reliability of the US scoring was excellent. US scores were moderately correlated with increasing age, but not with other demographic or disease activity measures, and the biological meaning of this finding is unclear. While the ongoing PAMPA study is actively recruiting for a planned enrollment of 350 participants, these preliminary findings suggest that appropriate patients are being enrolled. These data also underscore the need to better characterize clinical and imaging phenotypes of high-risk patients with psoriasis for subclinical soft tissue/structural changes that may foreshadow transition to PsA.
To cite this abstract in AMA style:
Haberman R, Moussavi S, Zhang Y, Catron S, Samuels J, Blank R, Toprover M, Hu J, Gong C, Piguet V, Tausk F, Yeung J, Neimann A, Gulliver W, Merola J, Ogdie A, Rahman P, Chakravarty S, Thiele R, Eder L, Ritchlin C, Scher J. Power Doppler Musculoskeletal Abnormalities in Patients with Psoriasis at High Risk of Progression to Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/power-doppler-musculoskeletal-abnormalities-in-patients-with-psoriasis-at-high-risk-of-progression-to-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/power-doppler-musculoskeletal-abnormalities-in-patients-with-psoriasis-at-high-risk-of-progression-to-psoriatic-arthritis/