Session Information
Date: Sunday, November 8, 2015
Title: Health Services Research Poster I: Diagnosis, Management and Treatment Strategies
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
Underreporting and inherent deficiencies are common
using spontaneous reports for post-marketing surveillance. The U.S. Food and
Drug Administration (FDA) has recommended sponsors to collect information from
multiple sources including creating or working with registries or other
observational data for post-marketing surveillance. For new costly drugs,
traditional registries are expensive to create, slow to recruit, and are
potentially subject to selection biases as to the types of patients enrolled.
The current study evaluated the feasibility of utilizing one or more healthcare
databases to support safety surveillance of a new biologic medication, with ustekinumab
(UST) as an example.
Methods:
Using patients from Medicare 2006-2012,
Marketscan 2008-2013, and Optum research database 2008-2013, we identified new
users of UST using both non-specific and or specific drug codes. New users were
defined as no previous use of UST with all available data. Eligible patients were
required to meet the following criteria: 1) ≥ 19 years of age; 2) ≥
1 one inpatient or dermatologist-given diagnosis code for psoriasis; 3) continuously
enrolled with medical and pharmacy coverage during the 6 months prior to UST initiation
and throughout follow up. We evaluated the accrual of new UST users by calendar
time in each data system.
Results:
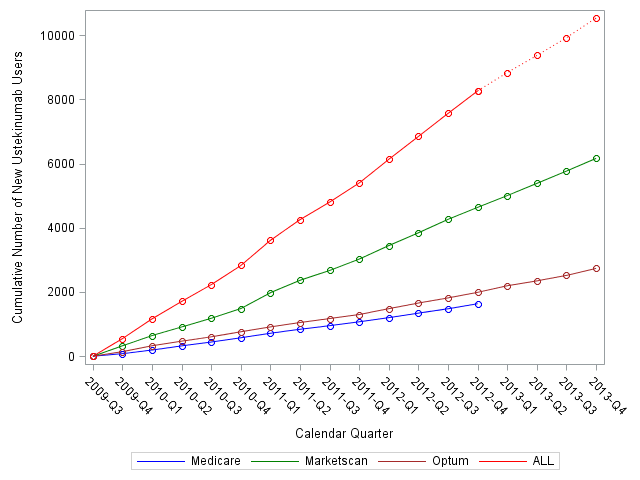
We identified 1636, 6159, and 2735 new UST
users in Medicare, Marketscan and Optum databases, respectively (Figure). These
patients had median follow-up of 1.5-2.5 years, and they were censored mainly because
our study observation period ended, and not because patients dis-enrolled from
the health plan. Marketscan provided the largest data source of new UST users,
whereas the number of new users was comparable between Medicare and Optum. Within
1 year of FDA licensure, approximately 3,000 new users were identified, and by
2 years, almost 6,000 users were identified. As of the end of 2013, more than
10,000 UST new users were available.
Conclusion:
Utilizing
one or more healthcare databases to support post-marketing surveillance for a
new biologic shortly after its licensure is feasible, and it yields relatively
large sample sizes to study uncommon safety events. This approach can
complement more traditional registry-based approaches to detect important
safety signals and maximize power for long term comparative safety analyses.
Figure:
Cumulative number of new Ustekinumab users in different data systems by
calendar quarter
To cite this abstract in AMA style:
Yun H, Yang S, Xie F, Chen L, Winthrop KL, Curtis JR. Potential Use of Healthcare Databases for Post-Marketing Surveillance Registry: an Example Using Ustekinumab [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/potential-use-of-healthcare-databases-for-post-marketing-surveillance-registry-an-example-using-ustekinumab/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/potential-use-of-healthcare-databases-for-post-marketing-surveillance-registry-an-example-using-ustekinumab/