Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Published data suggest no increased rate of flare of autoimmune inflammatory rheumatic diseases (AIIRD) after COVID-19 mRNA vaccination; however, the studies are limited by small sample size, short follow up or at risk of selection bias (voluntary physician reports or patient surveys). Therefore, our aim is to study flares of AIIRD within three months of the first dose of an anti-SARS-COV2 mRNA vaccine.
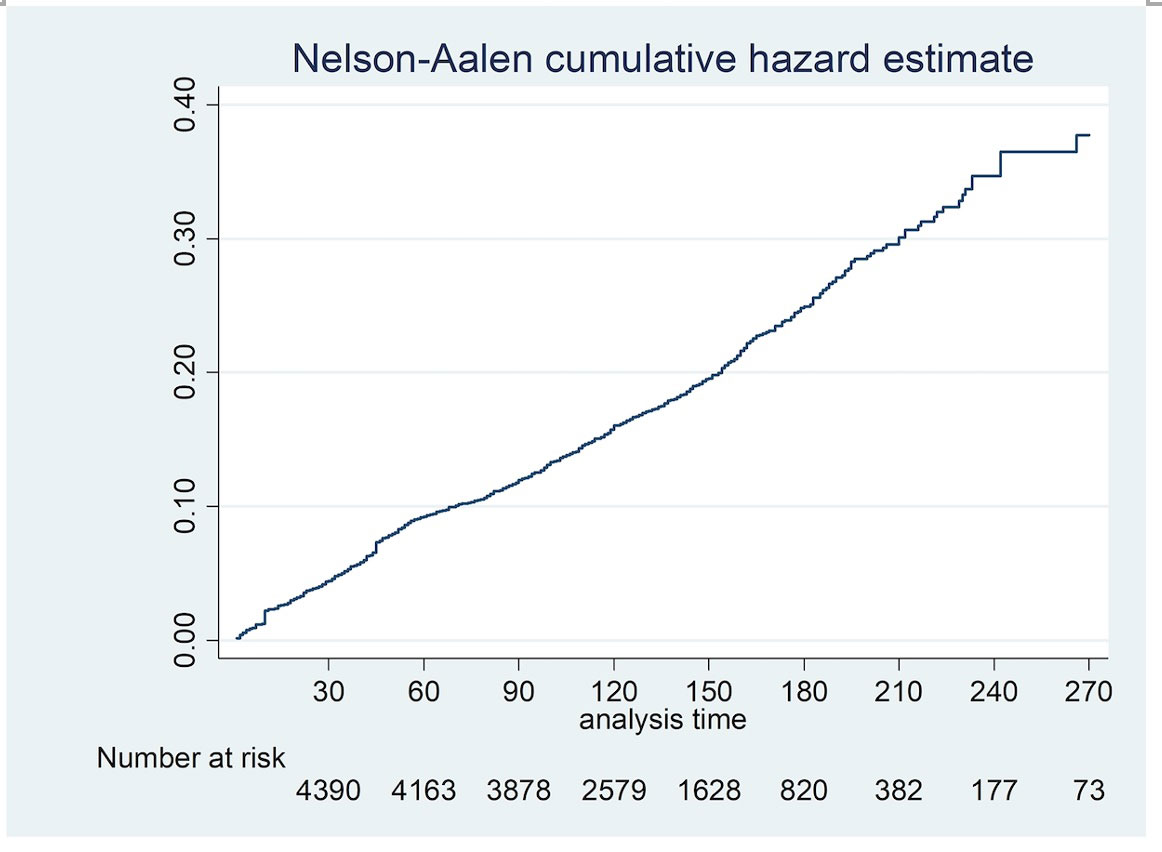
Methods: A retrospective cohort study of consecutive AIIRD patients ≥ 12 years old, across 8 public hospitals in Singapore who received at least one dose of an mRNA (Pfizer/BioNTech or Moderna) vaccine. Data were censored at the first post-vaccine clinic visit when the patient had flared or if ≥ three months had elapsed since the first dose of the vaccine, whichever came first. Predictors of flare were determined by Cox proportional hazards analysis and time to flare was examined using a Nelson Aalen cumulative hazard estimate (Figure 1).
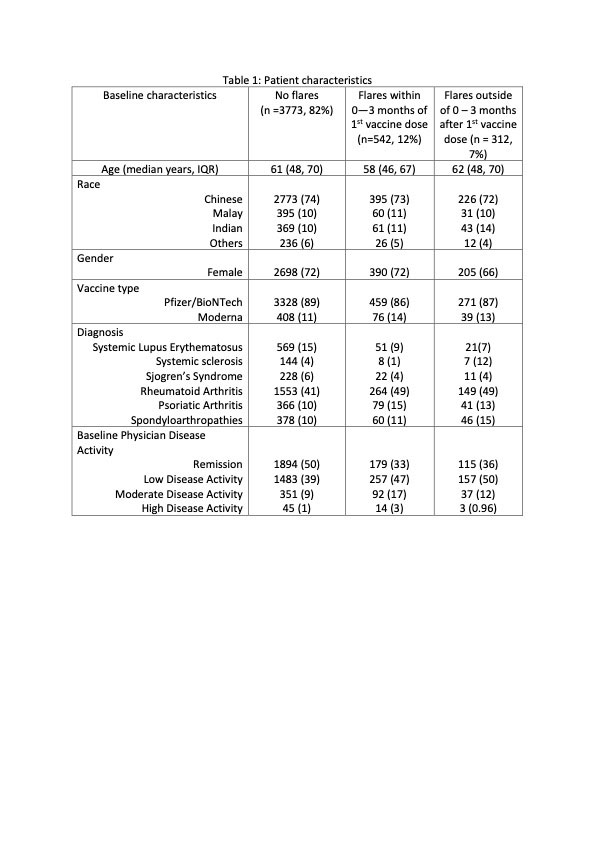
Results: 4627 patients (73.3% Chinese, 71.1% female) of median (IQR) age 61 (48, 70) years were included in the analysis (Table). 4058 (87.7%) had the Pfizer/BioNTech vaccine and 523 (11.3%) had Moderna, with a median (IQR) interval of 21 (21, 28) days between the two doses. The most common AIIRD diagnoses were Rheumatoid arthritis (1966, 42.4%), Psoriatic arthritis (486, 10.5%) and Systemic lupus erythematosus (SLE) (641, 13.8%). 2188 (47.3%) patients were in remission, 1897 (41%) in low disease activity, 480 (10.4%) in moderate disease activity and 62 (1.3%) in high disease activity. 431 (9.3%) were treated with biologics/ targeted disease modifying agents. Treatment was interrupted for vaccination in only 63 (1.4 %) patients. 45 (1%) patients had previous COVID-19 infection. 854 (18%) flares were recorded during 20,287 patient-months (4,5/100 patient-months, median (IQR) follow up duration 4.3 (3.4, 5.5) months), of which 542 (11.7%) patients flared in the 3-month period of interest. Median (IQR) time to flare was 40 (18, 56) days (Figure 1). 134 (24.7%) flares were mild and self-limiting, 333 (61.4%) were mild-moderate and 75 (13.8%) were severe. 393 (72.5%) of those who flared required escalation of treatment and 30 (5.5%) required hospital admission. 545 (11.8%) had improved disease activity after the vaccine.
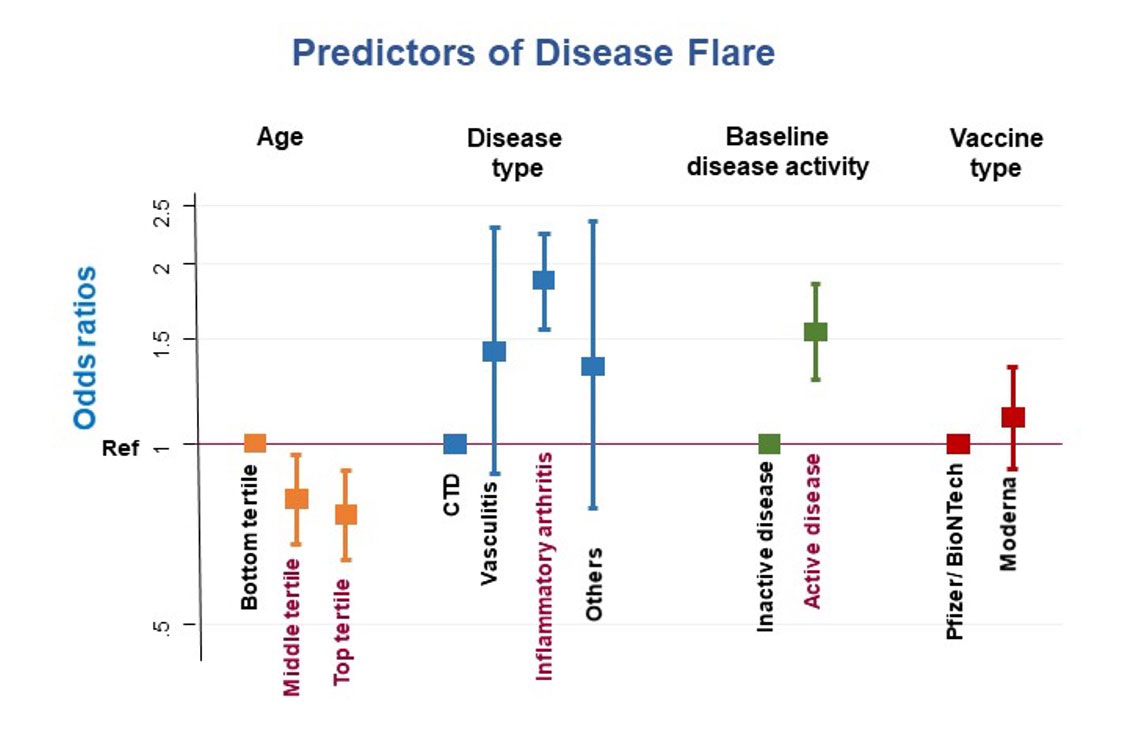
On multivariate Cox regression analysis, patients in the oldest age tertile [median (IQR) 72 (69, 77) years] were less likely to flare [HR 0.76 (95% CI 0.64, 0.90), p = 0.001] Patients with inflammatory arthritis (compared with connective tissue disease, vasculitis and others) and patients with baseline active disease were more likely to flare [HR 1.88 (95% CI 1.56, 2.25), p < 0.001 and 1.54 (95% CI 1.28, 1.85), p < 0.001 respectively] Gender, ethnicity and vaccine type were not significant predictors of flare. (Figure 2)
Conclusion: There was a moderately high rate of AIIRD flares after mRNA vaccination; however, there was no clustering of flares in the immediate post-vaccine period to suggest causality and severe flares were rare. Older patients were less likely to flare, while those with inflammatory arthritis and active disease at baseline were more likely to flare.
To cite this abstract in AMA style:
Ma M, Santosa A, Kong K, Xu C, Teng G, Tang J, Mak A, Tay F, Ng V, Koh J, Fong W, Chew L, Low A, law a, Poh Y, Yeo S, Leung Y, Goh W, Roslan N, Chuah T, Angkodjojo S, Arkachaisri T, Teh K, Phang K, Sriranganathan M, Tan t, Cheung P, Lahiri M. Post-mRNA Vaccine Flares in Autoimmune Inflammatory Rheumatic Diseases: Results from the COronavirus National Vaccine Registry for ImmuNe Diseases SINGapore (CONVIN-SING) [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/post-mrna-vaccine-flares-in-autoimmune-inflammatory-rheumatic-diseases-results-from-the-coronavirus-national-vaccine-registry-for-immune-diseases-singapore-convin-sing/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/post-mrna-vaccine-flares-in-autoimmune-inflammatory-rheumatic-diseases-results-from-the-coronavirus-national-vaccine-registry-for-immune-diseases-singapore-convin-sing/