Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
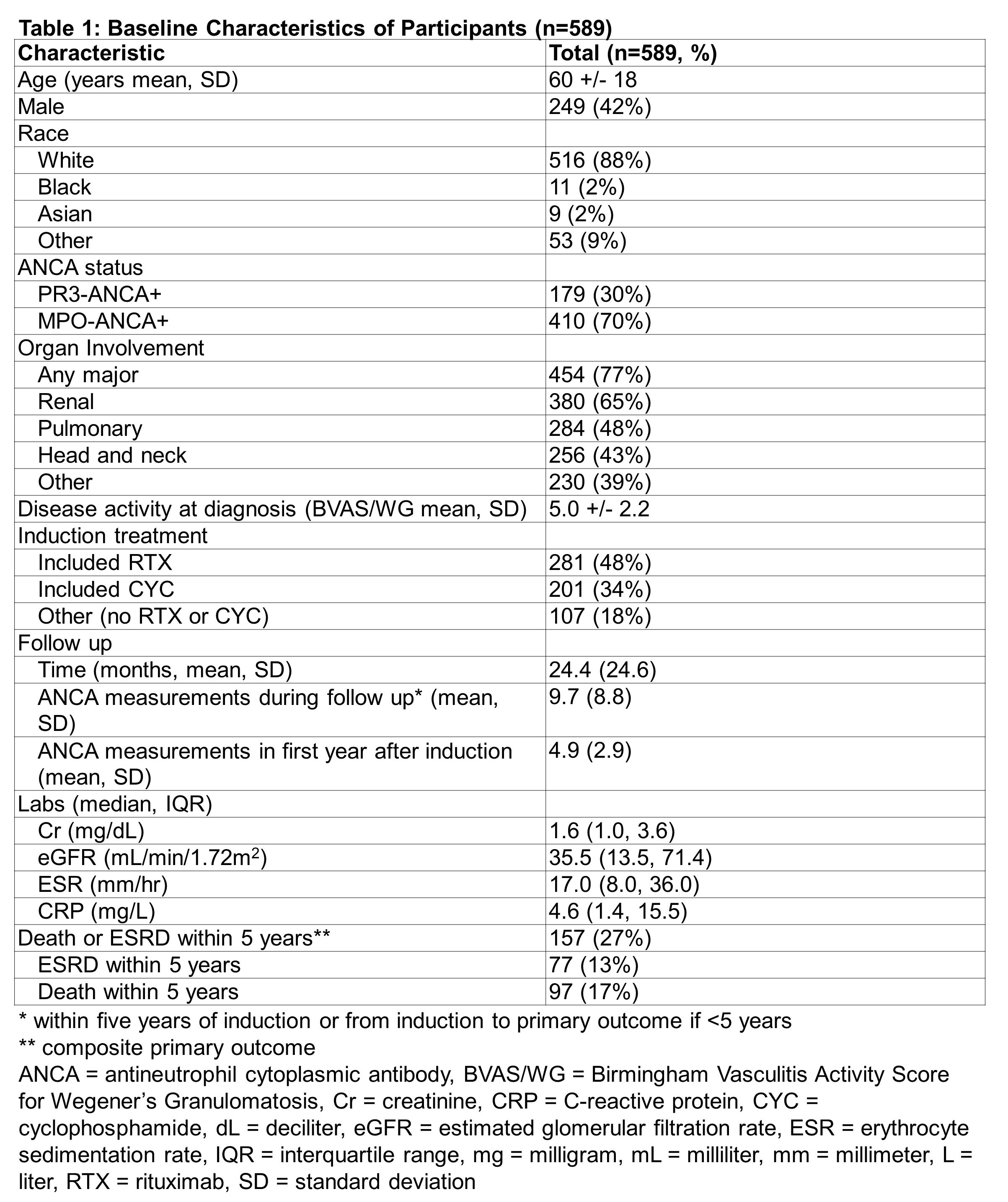
Background/Purpose: Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is associated with increased risk of end-stage renal disease (ESRD) and death. In most cases, circulating ANCA targeting proteinase-3 (PR3) or myeloperoxidase (MPO) are present and may be pathogenic. Studies of the association of post-treatment ANCA titers with future outcomes have yielded conflicting results. We evaluated the association of achieving a negative ANCA titer during the first year of treatment with ESRD and death.
Methods: Cases were obtained from a consecutive inception cohort of AAV patients who received care at a large multi-hospital system between 2002-2019. All cases were PR3- or MPO-ANCA+. ANCA titers were obtained from a single reference laboratory. Mortality data were obtained from the National Death Index and ESRD status from the United States Renal Data System. To address confounding and immortal-time bias, we performed a target trial emulation study to examine the association of post-induction ANCA titer with risk of ESRD or death using a previously described cloning, censoring and weighting approach. We designed a hypothetical trial with two management strategies: “achieve a negative titer” or “do not achieve negative titer” within 365 days of induction. “Clones” of each patient were included in each hypothetical management arm and censored when they violated the assigned strategy. The composite outcome – risk of ESRD or death within five years – was estimated using Cox models after accounting for informative censoring using inverse-probability-of-censoring weighting and adjusting for baseline covariates.
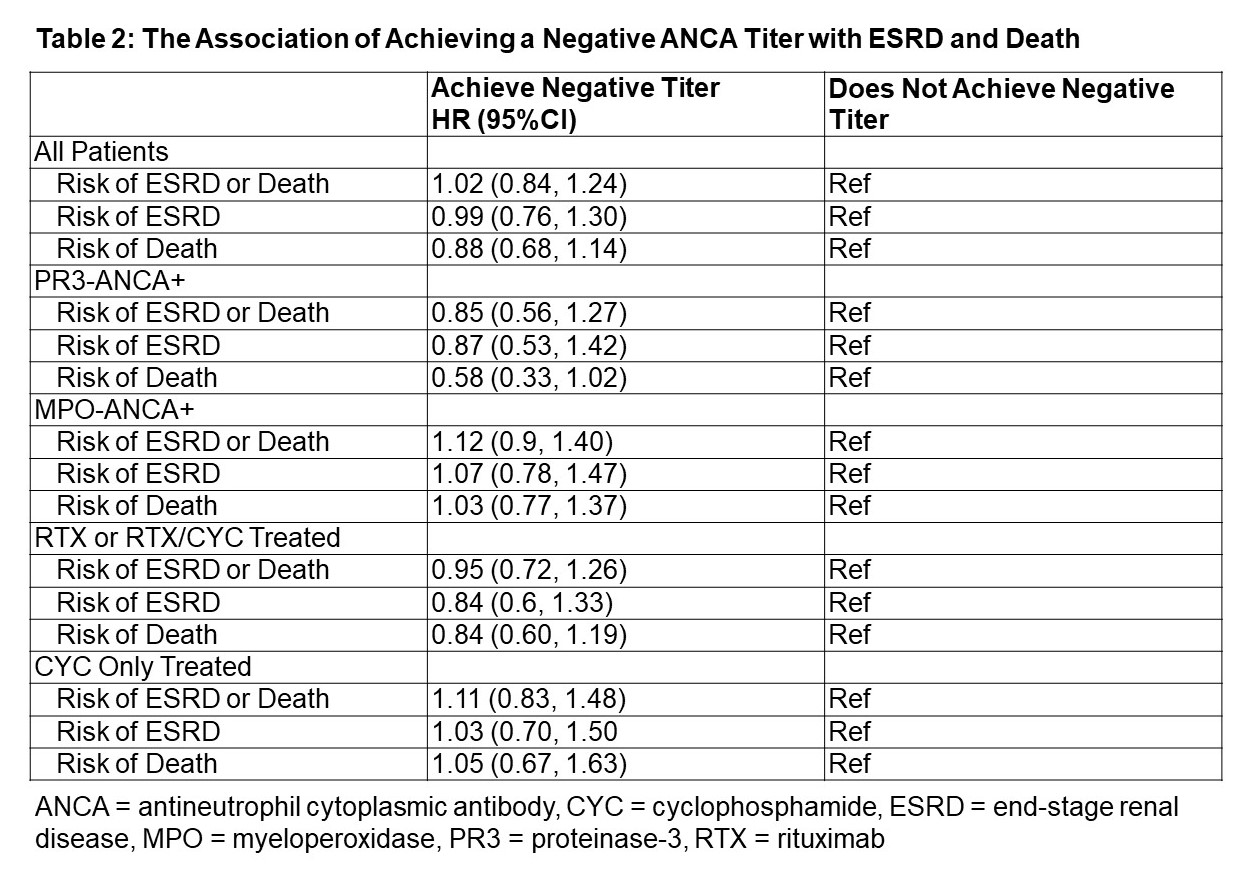
Results: The study included 589 patients (mean age: 60 years; 58% female; 88% white) with 24.4 + 24.6 months of mean follow up (Table 1). The majority were MPO-ANCA+ (70%) and had renal manifestations (65%). Rituximab (RTX)-based induction strategies were used in 48%. 32% achieved a negative titer within one year of induction. In the target trial, the HR for the primary outcome of ESRD or death was 1.02 (95%CI 0.84-1.24) in the group that achieved a negative titer compared to the group that did not (Table 2). In analyses stratified by remission induction strategy, the HR for ESRD or death was 0.95 (95%CI 0.72-1.26) in RTX-based and 1.11 (95%CI 0.83-1.48) in cyclophosphamide (CYC)-based regimen users when comparing those who achieved a negative titer vs those who did not. The HR for ESRD or death was 0.85 (95%CI 0.56-1.27) in the PR3-ANCA+ and 1.12 (95%CI 0.90-1.40) in the MPO-ANCA+ groups that achieved a negative titer. There was a trend toward lower risk of death among PR3-ANCA+ patients who achieved a negative titer (HR 0.58 [95%CI 0.33-1.02]).
Conclusion: In this target trial using a large cohort of AAV patients, achieving a negative ANCA titer within one year of induction was not associated with improved renal or mortality outcomes. This finding was seen in both RTX- and CYC-treated patients. There was a trend towards decreased risk of death in the PR3-ANCA+ patients who achieved a negative titer, although this did not reach statistical significance. These findings suggest that post-induction ANCA titers have limited ability to predict mortality and ESRD outcomes in AAV.
To cite this abstract in AMA style:
McDermott G, Fu X, Cook C, Stone J, Zhang Y, Wallace Z. Post-induction ANCA Titer Does Not Predict Mortality or Renal Outcomes: A Target Trial Emulation Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/post-induction-anca-titer-does-not-predict-mortality-or-renal-outcomes-a-target-trial-emulation-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/post-induction-anca-titer-does-not-predict-mortality-or-renal-outcomes-a-target-trial-emulation-study/