Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Tofacitinib is a novel oral Janus kinase (JAK) inhibitor for the treatment of rheumatoid arthritis (RA). Serious infections (requiring hospitalization or parenteral antibiotics; SIEs) have been reported at an incidence of approximately 3 events per 100 patient-years (pt-y) in tofacitinib-treated RA patients (pts). This retrospective analysis incorporating Phase (P) 2, 3 and long-term extension (LTE) safety data assessed the relationship between selected clinical factors and SIE occurrence.
Methods: Data from pts receiving tofacitinib were pooled from five randomized P2, five randomized P3 and two open-label LTE studies. Clinical factors in the analysis were a) age (≥65 years vs <65 years), b) diabetes status (yes/no), c) concomitant baseline glucocorticoid use (≥7.5 mg vs <7.5 mg), d) tofacitinib dose (as a continuous variable incorporating dose changes over time), e) prior treatment (inadequate responders; IR) with biologic vs non-biologic disease modifying anti-rheumatic drugs (DMARDs), f) pts experiencing a treated infection (i.e. infection requiring antimicrobial therapy) within the first 6 months of treatment vs none, and g) duration of disease (RA) at baseline (as a continuous variable). SIE data were analyzed using a Cox Proportional Hazards model with time dependent covariates (dose), with simultaneous inclusion of the above covariates. Separate interaction tests between tofacitinib dose and age, diabetes or glucocorticoid dose were performed. Results were expressed as hazard ratios with 95% confidence intervals (CIs).
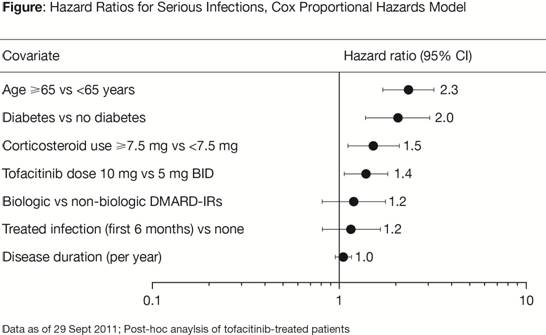
Results: Based upon the exclusion of 1 for 95% CI of hazard ratios, age (elderly), corticosteroid dose ≥7.5 mg, diabetes and tofacitinib dose were identified as independent factors associated with the risk of SIE (Figure). Elderly pts had an estimated 2.3 times increased risk of SIE. Pts with diabetes had 2 times increased risk of SIE, while a separate infections analysis irrespective of severity did not show noticeable differences (data on file). There was a 50% increase in SIE risk in pts receiving corticosteroid doses ≥7.5 mg and 40% increased risk of SIE with 10 mg vs 5 mg tofacitinib. Interaction tests did not show an increase in tofacitinib risk in the above sub-populations. Relative to non-biologic DMARD IRs, biologic DMARD IRs showed a hazard ratio of 1.2, with CIs including 1.
Conclusion: Identification of age, diabetes and corticosteroid dose as independent risk factors is consistent with reports from multiple RA pt databases (Listing et al., 2013; Strangfeld A, et al. 2011) of biologic DMARDs. Tofacitinib dose was an independent risk factor for SIEs. However, interaction tests suggest that the identified risk factors are reflective of underlying characteristics of the RA population, particularly DMARD-treated pts, rather than tofacitinib.
Disclosure:
J. J. Gomez-Reino,
Roche, UCB, MSD,
8,
Abbott, BMS, MSD, Pfizer Inc, Roche,
5,
Abbott, BMS, MSD, Pfizer Inc, Roche,
8;
A. Hazra,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
C. Fosser,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
S. Menon,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
S. H. Zwillich,
Pfizer Inc,
1,
Pfizer Inc,
3;
R. Riese,
Pfizer Inc,
1,
Pfizer Inc,
3;
S. Krishnaswami,
Pfizer Inc.,
1,
Pfizer Inc.,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/post-hoc-analysis-of-serious-infection-events-and-selected-clinical-factors-in-rheumatoid-arthritis-patients-treated-with-tofacitinib/